Ecology of antibiotic resistance genes: characterization of enterococci from houseflies collected in food settings
- PMID: 16751512
- PMCID: PMC1489584
- DOI: 10.1128/AEM.00034-06
Ecology of antibiotic resistance genes: characterization of enterococci from houseflies collected in food settings
Abstract
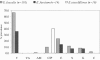
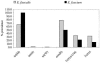
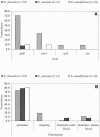
In this project, enterococci from the digestive tracts of 260 houseflies (Musca domestica L.) collected from five restaurants were characterized. Houseflies frequently (97% of the flies were positive) carried enterococci (mean, 3.1 x 10(3) CFU/fly). Using multiplex PCR, 205 of 355 randomly selected enterococcal isolates were identified and characterized. The majority of these isolates were Enterococcus faecalis (88.2%); in addition, 6.8% were E. faecium, and 4.9% were E. casseliflavus. E. faecalis isolates were phenotypically resistant to tetracycline (66.3%), erythromycin (23.8%), streptomycin (11.6%), ciprofloxacin (9.9%), and kanamycin (8.3%). Tetracycline resistance in E. faecalis was encoded by tet(M) (65.8%), tet(O) (1.7%), and tet(W) (0.8%). The majority (78.3%) of the erythromycin-resistant E. faecalis isolates carried erm(B). The conjugative transposon Tn916 and members of the Tn916/Tn1545 family were detected in 30.2% and 34.6% of the identified isolates, respectively. E. faecalis carried virulence genes, including a gelatinase gene (gelE; 70.7%), an aggregation substance gene (asa1; 33.2%), an enterococcus surface protein gene (esp; 8.8%), and a cytolysin gene (cylA; 8.8%). Phenotypic assays showed that 91.4% of the isolates with the gelE gene were gelatinolytic and that 46.7% of the isolates with the asa1 gene aggregated. All isolates with the cylA gene were hemolytic on human blood. This study showed that houseflies in food-handling and -serving facilities carry antibiotic-resistant and potentially virulent enterococci that have the capacity for horizontal transfer of antibiotic resistance genes to other bacteria.
Figures
References
-
- Aarestrup, F. M., Y. Agerso, P. Gerner-Smidt, M. Madsen, and L. B. Jensen. 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in E. faecalis and E. faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 37:127-137. - PubMed
-
- Aarestrup, F. M., A. M. Seyfarth, H.-D. Emborg, K. Pedersen, R. S. Hendriksen, and F. Bager. 2001. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 45:2054-2059. - PMC - PubMed
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

