Effect of P availability on temporal dynamics of carbon allocation and glomus intraradices high-affinity P transporter gene induction in arbuscular mycorrhiza
- PMID: 16751522
- PMCID: PMC1489668
- DOI: 10.1128/AEM.02154-05
Effect of P availability on temporal dynamics of carbon allocation and glomus intraradices high-affinity P transporter gene induction in arbuscular mycorrhiza
Abstract
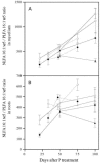
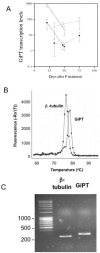
Arbuscular mycorrhizal (AM) fungi depend on a C supply from the plant host and simultaneously provide phosphorus to the colonized plant. We therefore evaluated the influence of external P on C allocation in monoxenic Daucus carota-Glomus intraradices cultures in an AM symbiosis. Fungal hyphae proliferated from a solid minimal medium containing colonized roots into a C-free liquid minimal medium with high or low P availability. Roots and hyphae were harvested periodically, and the flow of C from roots to fungus was measured by isotope labeling. We also measured induction of a G. intraradices high-affinity P transporter to estimate fungal P demand. The prevailing hypothesis is that high P availability reduces mycorrhizal fungal growth, but we found that C flow to the fungus was initially highest at the high P level. Only at later harvests, after 100 days of in vitro culture, were C flow and fungal growth limited at high P availability. Thus, AM fungi can benefit initially from P-enriched environments in terms of plant C allocation. As expected, the P transporter induction was significantly greater at low P availability and greatest in very young mycelia. We found no direct link between C flow to the fungus and the P transporter transcription level, which indicates that a good C supply is not essential for induction of the high-affinity P transporter. We describe a mechanism by which P regulates symbiotic C allocation, and we discuss how this mechanism may have evolved in a competitive environment.
Figures


Similar articles
-
The influence of external nitrogen on carbon allocation to Glomus intraradices in monoxenic arbuscular mycorrhiza.New Phytol. 2005 Dec;168(3):677-86. doi: 10.1111/j.1469-8137.2005.01532.x. New Phytol. 2005. PMID: 16313649
-
Phosphorus effects on metabolic processes in monoxenic arbuscular mycorrhiza cultures.Plant Physiol. 2002 Nov;130(3):1162-71. doi: 10.1104/pp.009639. Plant Physiol. 2002. PMID: 12427983 Free PMC article.
-
Carbon dynamics in mycorrhizal symbioses is linked to carbon costs and phosphorus benefits.FEMS Microbiol Ecol. 2010 Apr;72(1):125-31. doi: 10.1111/j.1574-6941.2009.00833.x. FEMS Microbiol Ecol. 2010. PMID: 20459516
-
Diet of Arbuscular Mycorrhizal Fungi: Bread and Butter?Trends Plant Sci. 2017 Aug;22(8):652-660. doi: 10.1016/j.tplants.2017.05.008. Epub 2017 Jun 13. Trends Plant Sci. 2017. PMID: 28622919 Review.
-
Nutrient Exchange and Regulation in Arbuscular Mycorrhizal Symbiosis.Mol Plant. 2017 Sep 12;10(9):1147-1158. doi: 10.1016/j.molp.2017.07.012. Epub 2017 Aug 3. Mol Plant. 2017. PMID: 28782719 Review.
Cited by
-
Ammonia-Oxidizing Archaea and Bacteria Differentially Contribute to Ammonia Oxidation in Sediments from Adjacent Waters of Rushan Bay, China.Front Microbiol. 2018 Feb 2;9:116. doi: 10.3389/fmicb.2018.00116. eCollection 2018. Front Microbiol. 2018. PMID: 29456526 Free PMC article.
-
Biotrophic transportome in mutualistic plant-fungal interactions.Mycorrhiza. 2013 Nov;23(8):597-625. doi: 10.1007/s00572-013-0496-9. Epub 2013 Apr 10. Mycorrhiza. 2013. PMID: 23572325 Review.
-
Distribution and abundance of archaeal and bacterial ammonia oxidizers in the sediments of the Dongjiang River, a drinking water supply for Hong Kong.Microbes Environ. 2013;28(4):457-65. doi: 10.1264/jsme2.me13066. Epub 2013 Nov 19. Microbes Environ. 2013. PMID: 24256973 Free PMC article.
-
An in vivo whole-plant experimental system for the analysis of gene expression in extraradical mycorrhizal mycelium.Mycorrhiza. 2017 Oct;27(7):659-668. doi: 10.1007/s00572-017-0779-7. Epub 2017 Jun 1. Mycorrhiza. 2017. PMID: 28573458
-
Fluorescent in situ RT-PCR to visualise the expression of a phosphate transporter gene from an ectomycorrhizal fungus.Mycorrhiza. 2007 Sep;17(6):487-494. doi: 10.1007/s00572-007-0127-4. Epub 2007 May 23. Mycorrhiza. 2007. PMID: 17520293
References
-
- Abbott, L. K., A. D. Robson, and G. De Boer. 1984. The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytol. 97:437-446.
-
- Bååth, E., and J. Spokes. 1989. The effect of added nitrogen and phosphorus on mycorrhizal growth response and infection in Allium schoenoprasum. Can. J. Bot. 67:3227-3232.
-
- Bago, B., C. Azcon-Aguilar, A. Goulet, and Y. Piché. 1998. Branched absorbing structures (BAS): a feature of the extraradical mycelium of symbiotic arbuscular mycorrhizal fungi. New Phytol. 139:375-388.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

