Quantification of in vitro and in vivo Cryptosporidium parvum infection by using real-time PCR
- PMID: 16751574
- PMCID: PMC1489663
- DOI: 10.1128/AEM.00189-06
Quantification of in vitro and in vivo Cryptosporidium parvum infection by using real-time PCR
Abstract
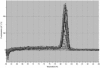
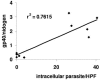
Established methods for quantifying experimental Cryptosporidium infection are highly variable and subjective. We describe a new technique using quantitative real-time PCR (qPCR) that can be used to measure in vitro and in vivo laboratory infections with Cryptosporidium. We show for the first time that qPCR permits absolute quantification of the parasite while simultaneously controlling for the amount of host tissue and correlates significantly with established methods of quantification in in vitro and in vivo laboratory models of infection.
Figures

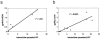
Similar articles
-
Two novel genomic DNA sequences as common diagnostic targets to detect Cryptosporidium hominis and Cryptosporidium parvum: Development of quantitative polymerase chain reaction assays, and clinical evaluation.Indian J Med Microbiol. 2020 Jul-Dec;38(3 & 4):430-439. doi: 10.4103/ijmm.IJMM_20_114. Indian J Med Microbiol. 2020. PMID: 33154258
-
Specific and quantitative detection and identification of Cryptosporidium hominis and C. parvum in clinical and environmental samples.Exp Parasitol. 2013 Sep;135(1):142-7. doi: 10.1016/j.exppara.2013.06.014. Epub 2013 Jul 6. Exp Parasitol. 2013. PMID: 23838581
-
Cryptosporidium Diagnostic Assays: Molecular Detection.Methods Mol Biol. 2020;2052:11-22. doi: 10.1007/978-1-4939-9748-0_2. Methods Mol Biol. 2020. PMID: 31452154
-
Laboratory diagnosis of Cryptosporidium parvum infection.Contrib Microbiol. 2000;6:33-49. doi: 10.1159/000060364. Contrib Microbiol. 2000. PMID: 10943506 Review. No abstract available.
-
[Research advances in the pathogenicity associated proteins of Cryptosporidium parvum].Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2004 Aug;22(4):253-5. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2004. PMID: 15587165 Review. Chinese. No abstract available.
Cited by
-
Dual Transcriptomics To Determine Gamma Interferon-Independent Host Response to Intestinal Cryptosporidium parvum Infection.Infect Immun. 2022 Feb 17;90(2):e0063821. doi: 10.1128/iai.00638-21. Epub 2021 Dec 20. Infect Immun. 2022. PMID: 34928716 Free PMC article.
-
A screening pipeline for antiparasitic agents targeting cryptosporidium inosine monophosphate dehydrogenase.PLoS Negl Trop Dis. 2010 Aug 10;4(8):e794. doi: 10.1371/journal.pntd.0000794. PLoS Negl Trop Dis. 2010. PMID: 20706578 Free PMC article.
-
Assessment of the potential occurrence of Cryptosporidium species in various water sources in Sharqia Governorate, Egypt.J Parasit Dis. 2024 Jun;48(2):358-369. doi: 10.1007/s12639-024-01675-1. Epub 2024 May 9. J Parasit Dis. 2024. PMID: 38840871 Free PMC article.
-
The Marine Compound Tartrolon E Targets the Asexual and Early Sexual Stages of Cryptosporidium parvum.Microorganisms. 2022 Nov 15;10(11):2260. doi: 10.3390/microorganisms10112260. Microorganisms. 2022. PMID: 36422330 Free PMC article.
-
Interferon-λ3 Promotes Epithelial Defense and Barrier Function Against Cryptosporidium parvum Infection.Cell Mol Gastroenterol Hepatol. 2019;8(1):1-20. doi: 10.1016/j.jcmgh.2019.02.007. Epub 2019 Mar 5. Cell Mol Gastroenterol Hepatol. 2019. PMID: 30849550 Free PMC article.
References
-
- Behera, A. K., C. M. Thorpe, J. M. Kidder, W. Smith, E. Hildebrand, and L. T. Hu. 2004. Borrelia burgdorferi-induced expression of matrix metalloproteinases from human chondrocytes requires mitogen-activated protein kinase and Janus kinase/signal transducer and activator of transcription signaling pathways. Infect. Immun. 72:2864-2871. - PMC - PubMed
-
- Bens, M., A. Bogdanova, F. Cluzeaud, L. Miquerol, S. Kerneis, J. P. Kraehenbuhl, A. Kahn, E. Pringault, and A. Vandewalle. 1996. Transimmortalized mouse intestinal cells (m-ICc12) that maintain a crypt phenotype. Am. J. Physiol. 270:C1666-C1674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

