Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder
- PMID: 16751797
- PMCID: PMC1751864
- DOI: 10.1038/sj.bjp.0706780
Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder
Abstract
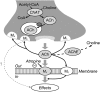
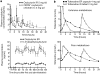
1. The effectiveness of antimuscarinic agents in the treatment of the overactive bladder (OAB) syndrome is thought to arise through blockade of bladder muscarinic receptors located on detrusor smooth muscle cells, as well as on nondetrusor structures. 2. Muscarinic M3 receptors are primarily responsible for detrusor contraction. Limited evidence exists to suggest that M2 receptors may have a role in mediating indirect contractions and/or inhibition of detrusor relaxation. In addition, there is evidence that muscarinic receptors located in the urothelium/suburothelium and on afferent nerves may contribute to the pathophysiology of OAB. Blockade of these receptors may also contribute to the clinical efficacy of antimuscarinic agents. 3. Although the role of muscarinic receptors in the bladder, other than M3 receptors, remains unclear, their role in other body systems is becoming increasingly well established, with emerging evidence supporting a wide range of diverse functions. Blockade of these functions by muscarinic receptor antagonists can lead to similarly diverse adverse effects associated with antimuscarinic treatment, with the range of effects observed varying according to the different receptor subtypes affected. 4. This review explores the evolving understanding of muscarinic receptor functions throughout the body, with particular focus on the bladder, gastrointestinal tract, eye, heart, brain and salivary glands, and the implications for drugs used to treat OAB. The key factors that might determine the ideal antimuscarinic drug for treatment of OAB are also discussed. Further research is needed to show whether the M3 selective receptor antagonists have any advantage over less selective drugs, in leading to fewer adverse events.
Figures

References
-
- ABDEL-RAHMAN A., SHETTY A.K., ABOU-DONIA M.B. Disruption of the blood–brain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-War syndrome. Neurobiol. Dis. 2002;10:306–326. - PubMed
-
- ABRAMS P., CARDOZO L., FALL M., GRIFFITHS D., ROSIER P., ULMSTEN U., VAN KERREBROECK P., VICTOR A., WEIN A. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol. Urodyn. 2002;21:167–178. - PubMed
-
- ANAGNOSTARAS S.G., MURPHY G.G., HAMILTON S.E., MITCHELL S.L., RAHNAMA N.P., NATHANSON N.M., SILVA A.J. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat. Neurosci. 2003;6:51–58. - PubMed
-
- ANDERSON R.U., MOBLEY D., BLANK B., SALTZSTEIN D., SUSSET J., BROWN J.S. Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence. J. Urol. 1999;161:1809–1812. - PubMed
-
- ANDERSSON K.-E. Clinical pharmacology of terodiline. Scand. J. Urol. Nephrol. Suppl. 1984;87:13–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

