Steroids, triterpenoids and molecular oxygen
- PMID: 16754609
- PMCID: PMC1578733
- DOI: 10.1098/rstb.2006.1837
Steroids, triterpenoids and molecular oxygen
Abstract
There is a close connection between modern-day biosynthesis of particular triterpenoid biomarkers and presence of molecular oxygen in the environment. Thus, the detection of steroid and triterpenoid hydrocarbons far back in Earth history has been used to infer the antiquity of oxygenic photosynthesis. This prompts the question: were these compounds produced similarly in the past? In this paper, we address this question with a review of the current state of knowledge surrounding the oxygen requirement for steroid biosynthesis and phylogenetic patterns in the distribution of steroid and triterpenoid biosynthetic pathways. The hopanoid and steroid biosynthetic pathways are very highly conserved within the bacterial and eukaryotic domains, respectively. Bacteriohopanepolyols are produced by a wide range of bacteria, and are methylated in significant abundance at the C2 position by oxygen-producing cyanobacteria. On the other hand, sterol biosynthesis is sparsely distributed in distantly related bacterial taxa and the pathways do not produce the wide range of products that characterize eukaryotes. In particular, evidence for sterol biosynthesis by cyanobacteria appears flawed. Our experiments show that cyanobacterial cultures are easily contaminated by sterol-producing rust fungi, which can be eliminated by treatment with cycloheximide affording sterol-free samples. Sterols are ubiquitous features of eukaryotic membranes, and it appears likely that the initial steps in sterol biosynthesis were present in their modern form in the last common ancestor of eukaryotes. Eleven molecules of O2 are required by four enzymes to produce one molecule of cholesterol. Thermodynamic arguments, optimization of function and parsimony all indicate that an ancestral anaerobic pathway is highly unlikely. The known geological record of molecular fossils, especially steranes and triterpanes, is notable for the limited number of structural motifs that have been observed. With a few exceptions, the carbon skeletons are the same as those found in the lipids of extant organisms and no demonstrably extinct structures have been reported. Furthermore, their patterns of occurrence over billion year time-scales correlate strongly with environments of deposition. Accordingly, biomarkers are excellent indicators of environmental conditions even though the taxonomic affinities of all biomarkers cannot be precisely specified. Biomarkers are ultimately tied to biochemicals with very specific functional properties, and interpretations of the biomarker record will benefit from increased understanding of the biological roles of geologically durable molecules.
Figures



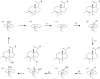
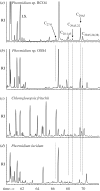
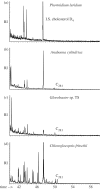
References
-
- Abe I, Rohmer M, Prestwich G.D. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem. Rev. 1993;93:2189–2206. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
