The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude
- PMID: 16754844
- PMCID: PMC1474012
- DOI: 10.1073/pnas.0603601103
The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude
Abstract
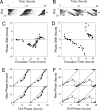
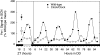
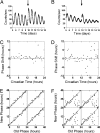
The mouse Clock gene encodes a basic helix-loop-helix-PAS transcription factor, CLOCK, that acts in concert with BMAL1 to form the positive elements of the circadian clock mechanism in mammals. The original Clock mutant allele is a dominant negative (antimorphic) mutation that deletes exon 19 and causes an internal deletion of 51 aa in the C-terminal activation domain of the CLOCK protein. Here we report that heterozygous Clock/+ mice exhibit high-amplitude phase-resetting responses to 6-h light pulses (Type 0 resetting) as compared with wild-type mice that have low amplitude (Type 1) phase resetting. The magnitude and time course of acute light induction in the suprachiasmatic nuclei of the only known light-induced core clock genes, Per1 and Per2, are not affected by the Clock/+ mutation. However, the amplitude of the circadian rhythms of Per gene expression are significantly reduced in Clock homozygous and heterozygous mutants. Rhythms of PER2::LUCIFERASE expression in suprachiasmatic nuclei explant cultures also are reduced in amplitude in Clock heterozygotes. The phase-response curves to changes in culture medium are Type 0 in Clock heterozygotes, but Type 1 in wild types, similar to that seen for light in vivo. The increased efficacy of resetting stimuli and decreased PER expression amplitude can be explained in a unified manner by a model in which the Clock mutation reduces circadian pacemaker amplitude in the suprachiasmatic nuclei.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
-
- Gekakis N., Staknis D., Nguyen H. B., Davis F. C., Wilsbacher L. D., King D. P., Takahashi J. S., Weitz C. J. Science. 1998;280:1564–1569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

