Essential and mutually compensatory roles of {alpha}-mannosidase II and {alpha}-mannosidase IIx in N-glycan processing in vivo in mice
- PMID: 16754854
- PMCID: PMC1474017
- DOI: 10.1073/pnas.0603248103
Essential and mutually compensatory roles of {alpha}-mannosidase II and {alpha}-mannosidase IIx in N-glycan processing in vivo in mice
Abstract
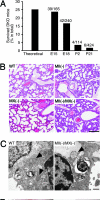
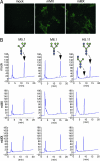
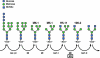
Many proteins synthesized through the secretory pathway receive posttranslational modifications, including N-glycosylation. alpha-Mannosidase II (MII) is a key enzyme converting precursor high-mannose-type N-glycans to matured complex-type structures. Previous studies showed that MII-null mice synthesize complex-type N-glycans, indicating the presence of an alternative pathway. Because alpha-mannosidase IIx (MX) is a candidate enzyme for this pathway, we asked whether MX functions in N-glycan processing by generating MII/MX double-null mice. Some double-nulls died between embryonic days 15.5 and 18.5, but most survived until shortly after birth and died of respiratory failure, which represents a more severe phenotype than that seen in single-nulls for either gene. Structural analysis of N-glycans revealed that double-nulls completely lack complex-type N-glycans, demonstrating a critical role for at least one of these enzymes for effective N-glycan processing. Recombinant mouse MX and MII showed identical substrate specificities toward N-glycan substrates, suggesting that MX is an isozyme of MII. Thus, either MII or MX can biochemically compensate for the deficiency of the other in vivo, and either of two is required for late embryonic and early postnatal development.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
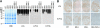


References
-
- Kornfeld R., Kornfeld S. Annu. Rev. Biochem. 1985;54:631–664. - PubMed
-
- Schachter H. Glycobiology. 1991;1:453–461. - PubMed
-
- Schachter H., Narasimhan S., Gleeson P., Vella G. Can. J. Biochem. Cell Biol. 1983;61:1049–1066. - PubMed
-
- Tulsiani D. R., Hubbard S. C., Robbins P. W., Touster O. J. Biol. Chem. 1982;257:3660–3668. - PubMed
-
- Moremen K. W., Trimble R. B., Herscovics A. Glycobiology. 1994;4:113–125. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

