Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice
- PMID: 16754860
- PMCID: PMC1482595
- DOI: 10.1073/pnas.0602956103
Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice
Abstract
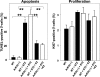
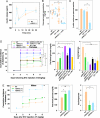
In diabetes, the death of insulin-producing beta-cells by apoptosis leads to insulin deficiency. The lower prevalence of diabetes in females suggests that female sex steroids protect from beta-cell injury. Consistent with this hypothesis, 17beta-estradiol (estradiol) manifests antidiabetic actions in humans and rodents. In addition, estradiol has antiapoptotic actions in cells that are mediated by the estrogen receptor-a (ERalpha), raising the prospect that estradiol antidiabetic function may be due, in part, to a protection of beta-cell apoptosis via ERalpha. To address this question, we have used mice that were rendered estradiol-deficient or estradiol-resistant by targeted disruption of aromatase (ArKO) or ERalpha (alphaERKO) respectively. We show here that in both genders, ArKO(-/-) mice are vulnerable to beta-cell apoptosis and prone to insulin-deficient diabetes after exposure to acute oxidative stress with streptozotocin. In these mice, estradiol treatment rescues streptozotocin-induced beta-cell apoptosis, helps sustain insulin production, and prevents diabetes. In vitro, in mouse pancreatic islets and beta-cells exposed to oxidative stress, estradiol prevents apoptosis and protects insulin secretion. Estradiol protection is partially lost in beta-cells and islets treated with an ERalpha antagonist and in alphaERKO islets. Accordingly, alphaERKO mice are no longer protected by estradiol and display a gender nonspecific susceptibility to oxidative injury, precipitating beta-cell apoptosis and insulin-deficient diabetes. Finally, the predisposition to insulin deficiency can be mimicked in WT mice by pharmacological inhibition of ERalpha by using the antagonist tamoxifen. This study demonstrates that estradiol, acting, at least in part, through ERalpha, protects beta-cells from oxidative injury and prevents diabetes in mice of both genders.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



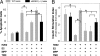
References
-
- Mathis D., Vence L., Benoist C. Nature. 2001;414:792–798. - PubMed
-
- Robertson R. P., Harmon J., Tran P. O., Tanaka Y., Takahashi H. Diabetes. 2003;52:581–587. - PubMed
-
- Wild S., Roglic G., Green A., Sicree R., King H. Diabetes Care. 2004;27:1047–1053. - PubMed
-
- Gale E. A., Gillespie K. M. Diabetologia. 2001;44:3–15. - PubMed
-
- Mauvais-Jarvis F., Sobngwi E., Porcher R., Riveline J. P., Kevorkian J. P., Vaisse C., Charpentier G., Guillausseau P. J., Vexiau P., Gautier J. F. Diabetes. 2004;53:645–653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

