Stem cell niches in the adult mouse heart
- PMID: 16754876
- PMCID: PMC1474010
- DOI: 10.1073/pnas.0600635103
Stem cell niches in the adult mouse heart
Abstract
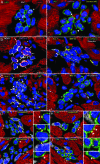
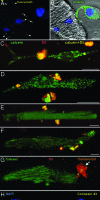
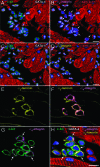
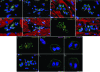
Cardiac stem cells (CSCs) have been identified in the adult heart, but the microenvironment that protects the slow-cycling, undifferentiated, and self-renewing CSCs remains to be determined. We report that the myocardium possesses interstitial structures with the architectural organization of stem cell niches that harbor long-term BrdU-retaining cells. The recognition of long-term label-retaining cells provides functional evidence of resident CSCs in the myocardium, indicating that the heart is an organ regulated by a stem cell compartment. Cardiac niches contain CSCs and lineage-committed cells, which are connected to supporting cells represented by myocytes and fibroblasts. Connexins and cadherins form gap and adherens junctions at the interface of CSCs-lineage-committed cells and supporting cells. The undifferentiated state of CSCs is coupled with the expression of alpha(4)-integrin, which colocalizes with the alpha(2)-chain of laminin and fibronectin. CSCs divide symmetrically and asymmetrically, but asymmetric division predominates, and the replicating CSC gives rise to one daughter CSC and one daughter committed cell. By this mechanism of growth kinetics, the pool of primitive CSCs is preserved, and a myocyte progeny is generated together with endothelial and smooth muscle cells. Thus, CSCs regulate myocyte turnover that is heterogeneous across the heart, faster at the apex and atria, and slower at the base-midregion of the ventricle.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Chien K. R. Nature. 2004;428:607–608. - PubMed
-
- Leri A., Kajstura J., Anversa P. Physiol. Rev. 2005;85:1373–1416. - PubMed
-
- Hierlihy A. M., Seale P., Lobe C. G., Rudnicki M. A., Megeney L. A. FEBS Lett. 2002;530:239–243. - PubMed
-
- Beltrami A. P., Barlucchi L., Torella D., Baker M., Limana F., Chimenti S., Kasahara H., Rota M., Musso E., Urbanek K., et al. Cell. 2003;114:763–776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

