Cytosolic inositol 1,4,5-trisphosphate dynamics during intracellular calcium oscillations in living cells
- PMID: 16754959
- PMCID: PMC2063891
- DOI: 10.1083/jcb.200512141
Cytosolic inositol 1,4,5-trisphosphate dynamics during intracellular calcium oscillations in living cells
Abstract
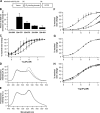
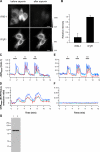
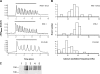
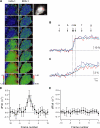
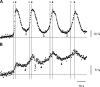
We developed genetically encoded fluorescent inositol 1,4,5-trisphosphate (IP3) sensors that do not severely interfere with intracellular Ca2+ dynamics and used them to monitor the spatiotemporal dynamics of both cytosolic IP3 and Ca2+ in single HeLa cells after stimulation of exogenously expressed metabotropic glutamate receptor 5a or endogenous histamine receptors. IP3 started to increase at a relatively constant rate before the pacemaker Ca2+ rise, and the subsequent abrupt Ca2+ rise was not accompanied by any acceleration in the rate of increase in IP3. Cytosolic [IP3] did not return to its basal level during the intervals between Ca2+ spikes, and IP3 gradually accumulated in the cytosol with a little or no fluctuations during cytosolic Ca2+ oscillations. These results indicate that the Ca2+ -induced regenerative IP3 production is not a driving force of the upstroke of Ca2+ spikes and that the apparent IP3 sensitivity for Ca2+ spike generation progressively decreases during Ca2+ oscillations.
Figures


References
-
- Allbritton, N.L., T. Meyer, and L. Stryer. 1992. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 258:1812–1815. - PubMed
-
- Berridge, M.J., and G. Dupont. 1994. Spatial and temporal signalling by calcium. Curr. Opin. Cell Biol. 6:267–274. - PubMed
-
- Berridge, M.J., P. Lipp, and M.D. Bootman. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21. - PubMed
-
- Bootman, M.D., and M.J. Berridge. 1996. Subcellular Ca2+ signals underlying waves and graded responses in HeLa cells. Curr. Biol. 6:855–865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

