Resonance Raman Structural Evidence that the Cis-to-Trans Isomerization in Rhodopsin Occurs in Femtoseconds
- PMID: 16755302
- PMCID: PMC1473983
- DOI: 10.1021/jp001236s
Resonance Raman Structural Evidence that the Cis-to-Trans Isomerization in Rhodopsin Occurs in Femtoseconds
Abstract
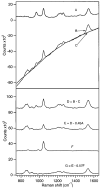
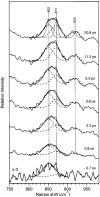
Picosecond time-resolved resonance Raman spectroscopy is used to probe the structural changes of rhodopsin's retinal chromophore as the cis-to-trans isomerization reaction occurs that initiates vision. Room-temperature resonance Raman spectra of rhodopsin's photoproduct with time delays from -0.7 to 20.8 ps are measured using 2.2 ps, 480 nm pump and 1.5 ps, 600 nm probe pulses. Hydrogen-out-of-plane (HOOP) modes at 852, 871, and 919 cm(-1), fingerprint peaks at 1272, 1236, 1211, and 1166 cm(-1), and a broad red-shifted ethylenic band at 1530 cm(-1) are present at the earliest positive pump-probe time delay of 0.8 ps, indicating that the chromophore is already in a strained, all-trans configuration. Kinetic analyses of both the HOOP and ethylenic regions of the photoproduct spectra reveal that these features grow in with fast ( approximately 200 fs) and slow ( approximately 2-3 ps) components. These data provide the first structural evidence that photorhodopsin has a thermally unrelaxed, torsionally strained all-trans chromophore within approximately 1 ps, and possibly within 200 fs, of photon absorption. Following this ultrafast product formation, the all-trans chromophore cools and conformationally relaxes within a few picoseconds to form bathorhodopsin. This cooling process is revealed as an ethylenic frequency blue-shift of 6 cm(-1) (tau approximately 3.5 ps) as well as an ethylenic width narrowing (tau approximately 2 ps). The ultrafast production of photorhodopsin is likely accompanied by an impulsively driven, localized protein response. More delocalized protein modes are unable to relax on this ultrafast time scale enabling the chromophore-protein complex to store the large amounts of photon energy (30-35 kcal/mol) that are subsequently used to drive activating protein conformational changes.
Figures








References
-
- Fleming GR, van Grondelle R. Curr. Opin. Struct. Biol. 1997;7:738–748. - PubMed
-
- Genick U, Borgstahl G, Kingman N, Zhong R, Pradervand C, Burke P, Srajer V, Teng T-Y, Schildkamp W, Mcree D, Moffat K, Getzoff E. Science. 1997;275:1471–1475. - PubMed
-
- Hoff WD, Xie A, van Stokkum IHM, Tang X-J, Gural J, Kroon AR, Hellingwerf KJ. Biochemistry. 1999;38:1009–1017. - PubMed
-
- Haupts U, Tittor J, Oesterhelt D. Annu. Rev. Biophys. Biomol. Struct. 1999;28:367–399. - PubMed
-
- Kochendoerfer GG, Mathies RA. Isr. J. Chem. 1995;35:211–226.
Grants and funding
LinkOut - more resources
Full Text Sources
