Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54
- PMID: 16756390
- PMCID: PMC1475773
- DOI: 10.1371/journal.pbio.0040210
Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54
Abstract
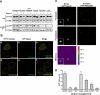
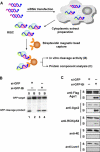
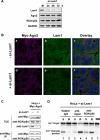
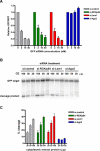
RNA interference is triggered by double-stranded RNA that is processed into small interfering RNAs (siRNAs) by Dicer enzyme. Endogenously, RNA interference triggers are created from small noncoding RNAs called microRNAs (miRNAs). RNA-induced silencing complexes (RISC) in human cells can be programmed by exogenously introduced siRNA or endogenously expressed miRNA. siRNA-programmed RISC (siRISC) silences expression by cleaving a perfectly complementary target mRNA, whereas miRNA-induced silencing complexes (miRISC) inhibits translation by binding imperfectly matched sequences in the 3' UTR of target mRNA. Both RISCs contain Argonaute2 (Ago2), which catalyzes target mRNA cleavage by siRISC and localizes to cytoplasmic mRNA processing bodies (P-bodies). Here, we show that RCK/p54, a DEAD box helicase, interacts with argonaute proteins, Ago1 and Ago2, in affinity-purified active siRISC or miRISC from human cells; directly interacts with Ago1 and Ago2 in vivo, facilitates formation of P-bodies, and is a general repressor of translation. Disrupting P-bodies by depleting Lsm1 did not affect RCK/p54 interactions with argonaute proteins and its function in miRNA-mediated translation repression. Depletion of RCK/p54 disrupted P-bodies and dispersed Ago2 throughout the cytoplasm but did not significantly affect siRNA-mediated RNA functions of RISC. Depleting RCK/p54 released general, miRNA-induced, and let-7-mediated translational repression. Therefore, we propose that translation repression is mediated by miRISC via RCK/p54 and its specificity is dictated by the miRNA sequence binding multiple copies of miRISC to complementary 3' UTR sites in the target mRNA. These studies also suggest that translation suppression by miRISC does not require P-body structures, and location of miRISC to P-bodies is the consequence of translation repression.
Figures



Comment in
-
Characterizing a key player in micro, but not small, RNA interference.PLoS Biol. 2006 Jul;4(7):e238. doi: 10.1371/journal.pbio.0040238. Epub 2006 Jun 13. PLoS Biol. 2006. PMID: 20076607 Free PMC article. No abstract available.
References
-
- Wienholds E, Plasterk RH. MicroRNA function in animal development. FEBS Lett. 2005;579:5911–5922. - PubMed
-
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. - PubMed
-
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Filipowicz W. RNAi: The nuts and bolts of the RISC machine. Cell. 2005;122:17–20. - PubMed
-
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans . Nature. 1998;391:806–811. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

