The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome
- PMID: 16756718
- PMCID: PMC1600674
- DOI: 10.1593/neo.05844
The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome
Abstract
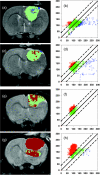
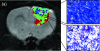
Functional diffusion map (fDM) has been recently reported as an early and quantitative biomarker of clinical brain tumor treatment outcome. This approach spatially maps and quantifies treatment-induced changes in tumor water diffusion values resulting from alterations in cell density/cell membrane function and microenvironment. This current study was designed to evaluate the capability of fDM for preclinical evaluation of dose escalation studies and to determine if these changes were correlated with outcome measures (cell kill and overall survival). Serial T2-weighted were carried out on rodents with orthotopically implanted 9L brain tumors receiving three doses of 1,3-bis(2-chloroethyl)-1-nitrosourea (6.65, 13.3, and 26.6 mg/kg, i.p.). All images were coregistered to baseline T2-weighted images for fDM analysis. Analysis of tumor fDM data on day 4 posttreatment detected dose-dependent changes in tumor diffusion values, which were also found to be spatially dependent. Histologic analysis of treated tumors confirmed spatial changes in cellularity as observed by fDM. Early changes in tumor diffusion values were found to be highly correlative with drug dose and independent biologic outcome measures (cell kill and survival). Therefore, The fDM imaging biomarker for early prediction of treatment efficacy can be used in the drug development process.
Figures


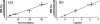
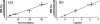
References
-
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. - PubMed
-
- Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res. 1997;3:1457–1466. - PubMed
-
- Ross BD, Stegman LD, Chenevert TL, Rehemtulla A. The role of magnetic resonance in the evaluation of cancer therapeutics. Clin Cancer Res. 1999;5:3870s–3871s.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
