Fluorescent nanoparticle uptake for brain tumor visualization
- PMID: 16756722
- PMCID: PMC1600680
- DOI: 10.1593/neo.05751
Fluorescent nanoparticle uptake for brain tumor visualization
Abstract
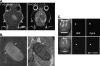
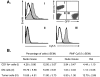
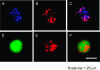
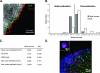
Accurate delineation of tumor margins is vital to the successful surgical resection of brain tumors. We have previously developed a multimodal nanoparticle CLIO-Cy5.5, which is detectable by both magnetic resonance imaging and fluorescence, to assist in intraoperatively visualizing tumor boundaries. Here we examined the accuracy of tumor margin determination of orthotopic tumors implanted in hosts with differing immune responses to the tumor. Using a nonuser-based signal intensity method applied to fluorescent micrographs of 9L gliosarcoma green fluorescent protein (GFP) tumors, mean overestimations of 2 and 24 microm were obtained using Cy5.5 fluorescence, compared to the true tumor margin determined by GFP fluorescence, in nude mice and rats, respectively. To resolve which cells internalized the nanoparticle and to quantitate degree of uptake, tumors were disaggregated and cells were analyzed by flow cytometry and fluorescence microscopy. Nanoparticle uptake was seen in both CD11b+ cells (representing activated microglia and macrophages) and tumor cells in both animal models by both methods. CD11b+ cells were predominantly found at the tumor margin in both hosts, but were more pronounced at the margin in the rat model. Additional metastatic (CT26 colon) and primary (Gli36 glioma) brain tumor models likewise demonstrated that the nanoparticle was internalized both by tumor cells and by host cells. Together, these observations suggest that fluorescent nanoparticles provide an accurate method of tumor margin estimation based on a combination of tumor cell and host cell uptake for primary and metastatic tumors in animal model systems and offer potential for clinical translation.
Figures

References
-
- Winger MJ, Macdonald DR, Cairncross JG. Supratentorial anaplastic gliomas in adults. The prognostic importance of extent of resection and prior low-grade glioma. J Neurosurg. 1989;71:487–493. - PubMed
-
- Bucci MK, Maity A, Janss AJ, Belasco JB, Fisher MJ, Tochner ZA, Rorke L, Sutton LN, Phillips PC, Shu HK. Near complete surgical resection predicts a favorable outcome in pediatric patients with nonbrainstem, malignant gliomas: results from a single center in the magnetic resonance imaging era. Cancer. 2004;101:817–824. - PubMed
-
- Moore GE. Fluorescein as an agent in the differentiation of normal and malignant tissues. Science. 1947;106:130–131. - PubMed
-
- Haglund MM, Hochman DW, Spence AM, Berger MS. Enhanced optical imaging of rat gliomas and tumor margins. Neurosurgery. 1994;35:930–940. discussion 940-931. - PubMed
-
- Haglund MM, Berger MS, Hochman DW. Enhanced optical imaging of human gliomas and tumor margins. Neurosurgery. 1996;38:308–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
