Thermodynamics reveal that helix four in the NLS of NF-kappaB p65 anchors IkappaBalpha, forming a very stable complex
- PMID: 16756995
- PMCID: PMC2680085
- DOI: 10.1016/j.jmb.2006.05.014
Thermodynamics reveal that helix four in the NLS of NF-kappaB p65 anchors IkappaBalpha, forming a very stable complex
Abstract
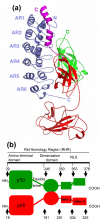
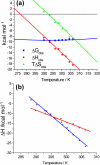
IkappaBalpha is an ankyrin repeat protein that inhibits NF-kappaB transcriptional activity by sequestering NF-kappaB outside of the nucleus in resting cells. We have characterized the binding thermodynamics and kinetics of the IkappaBalpha ankyrin repeat domain to NF-kappaB(p50/p65) using surface plasmon resonance (SPR) and isothermal titration calorimetry (ITC). SPR data showed that the IkappaBalpha and NF-kappaB associate rapidly but dissociate very slowly, leading to an extremely stable complex with a K(D,obs) of approximately 40 pM at 37 degrees C. As reported previously, the amino-terminal DNA-binding domain of p65 contributes little to the overall binding affinity. Conversely, helix four of p65, which forms part of the nuclear localization sequence, was essential for high-affinity binding. This was surprising, given the small size of the binding interface formed by this part of p65. The NF-kappaB(p50/p65) heterodimer and p65 homodimer bound IkappaBalpha with almost indistinguishable thermodynamics, except that the NF-kappaB p65 homodimer was characterized by a more favorable DeltaH(obs) relative to the NF-kappaB(p50/p65) heterodimer. Both interactions were characterized by a large negative heat capacity change (DeltaC(P,obs)), approximately half of which was contributed by the p65 helix four that was necessary for tight binding. This could not be accounted for readily by the small loss of buried non-polar surface area and we hypothesize that the observed effect is due to additional folding of some regions of the complex.
Figures





Similar articles
-
The IkappaBalpha/NF-kappaB complex has two hot spots, one at either end of the interface.Protein Sci. 2008 Dec;17(12):2051-8. doi: 10.1110/ps.037481.108. Epub 2008 Sep 29. Protein Sci. 2008. PMID: 18824506 Free PMC article.
-
Detection of a ternary complex of NF-kappaB and IkappaBalpha with DNA provides insights into how IkappaBalpha removes NF-kappaB from transcription sites.Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1367-72. doi: 10.1073/pnas.1014323108. Epub 2011 Jan 10. Proc Natl Acad Sci U S A. 2011. PMID: 21220295 Free PMC article.
-
The RelA nuclear localization signal folds upon binding to IκBα.J Mol Biol. 2011 Jan 21;405(3):754-64. doi: 10.1016/j.jmb.2010.10.055. Epub 2010 Nov 19. J Mol Biol. 2011. PMID: 21094161 Free PMC article.
-
Solvent exposed non-contacting amino acids play a critical role in NF-kappaB/IkappaBalpha complex formation.J Mol Biol. 2002 Dec 6;324(4):587-97. doi: 10.1016/s0022-2836(02)01149-x. J Mol Biol. 2002. PMID: 12460563
-
Molecular mechanisms of system control of NF-kappaB signaling by IkappaBalpha.Biochemistry. 2010 Mar 2;49(8):1560-7. doi: 10.1021/bi901948j. Biochemistry. 2010. PMID: 20055496 Free PMC article. Review.
Cited by
-
The TCL1A oncoprotein interacts directly with the NF-kappaB inhibitor IkappaB.PLoS One. 2009 Aug 10;4(8):e6567. doi: 10.1371/journal.pone.0006567. PLoS One. 2009. PMID: 19668332 Free PMC article.
-
Predicting coupling limits from an experimentally determined energy landscape.Proc Natl Acad Sci U S A. 2007 Mar 20;104(12):4907-12. doi: 10.1073/pnas.0608756104. Epub 2007 Mar 14. Proc Natl Acad Sci U S A. 2007. PMID: 17360387 Free PMC article.
-
Exclusivity and Compensation in NFκB Dimer Distributions and IκB Inhibition.Biochemistry. 2019 May 28;58(21):2555-2563. doi: 10.1021/acs.biochem.9b00008. Epub 2019 May 14. Biochemistry. 2019. PMID: 31033276 Free PMC article.
-
The IkappaBalpha/NF-kappaB complex has two hot spots, one at either end of the interface.Protein Sci. 2008 Dec;17(12):2051-8. doi: 10.1110/ps.037481.108. Epub 2008 Sep 29. Protein Sci. 2008. PMID: 18824506 Free PMC article.
-
Functional importance of stripping in NFκB signaling revealed by a stripping-impaired IκBα mutant.Proc Natl Acad Sci U S A. 2017 Feb 21;114(8):1916-1921. doi: 10.1073/pnas.1610192114. Epub 2017 Feb 6. Proc Natl Acad Sci U S A. 2017. PMID: 28167786 Free PMC article.
References
-
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Ann. Rev. Immunol. 2000;18:621–63. - PubMed
-
- Baldwin AS. The NF-kappa-B and I-kappa-B proteins: New discoveries and insights. Ann. Rev. Immunol. 1996;87:13–20. - PubMed
-
- Baltimore D, Alcarno E, Hoffmann A, Stankovski I. NF-kB's many facets. FASEB J. 1999;13:A1429.
-
- Gerondakis S, Grossmann M, Nakamura Y, Pohl T, Grumont R. Genetic approaches in mice to understand Rel/NF-kappaB and IkappaB function: transgenics and knockouts. Oncogene. 1999;18:6888–95. - PubMed
-
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Ann. Rev. Immunol. 1998;16:225–60. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

