An in-house RD1-based enzyme-linked immunospot-gamma interferon assay instead of the tuberculin skin test for diagnosis of latent Mycobacterium tuberculosis infection
- PMID: 16757583
- PMCID: PMC1489403
- DOI: 10.1128/JCM.02265-05
An in-house RD1-based enzyme-linked immunospot-gamma interferon assay instead of the tuberculin skin test for diagnosis of latent Mycobacterium tuberculosis infection
Abstract
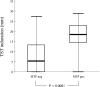
Identification of individuals infected with Mycobacterium tuberculosis is essential for the control of tuberculosis (TB). The specificity of the currently used tuberculin skin test (TST) is poor because of the broad antigenic cross-reactivity of purified protein derivative (PPD) with BCG vaccine strains and environmental mycobacteria. Both ESAT-6 and CFP-10, two secretory proteins that are highly specific for M. tuberculosis complex, elicit strong T-cell responses in subjects with TB. Using an enzyme-linked immunospot (ELISPOT)-IFN-gamma assay and a restricted pool of peptides derived from ESAT-6 and CFP-10, we have previously demonstrated a high degree of specificity and sensitivity of the test for the diagnosis of TB. Here, 119 contacts of individuals with contagious TB who underwent TST and the ELISPOT-IFN-gamma assay were consecutively recruited. We compared the efficacy of the two tests in detecting latent TB infection and defined a more appropriate TST cutoff point. There was little agreement between the tests (k = 0.33, P < 0.0001): 53% of the contacts with a positive TST were ELISPOT negative, and 7% with a negative TST were ELISPOT positive. Furthermore, respectively 76 and 59% of the ELISPOT-negative contacts responded in vitro to BCG and PPD, suggesting that most of them were BCG vaccinated or infected with nontuberculous mycobacteria. The number of spot-forming cells significantly correlated with TST induration (P < 0.0001). Our in-house ELISPOT assay based on a restricted pool of highly selected peptides is more accurate than TST for identifying individuals with latent TB infection and could improve chemoprophylaxis for the control of TB.
Figures

References
-
- American Thoracic Society. 2000. Targeted tuberculin testing and treatment of latent tuberculosis infection. Joint statement of the American Thoracic Society and the Centers for Disease Control and Prevention, endorsed by the Council of the Infectious Diseases Society of America in September 1999. Am. J. Respir. Crit. Care Med. 161:S221-S247. - PubMed
-
- American Thoracic Society. 2000. Diagnostic standards and classification of tuberculosis in adults and children. Joint statement of the American Thoracic Society and the Centers for Disease Control and Prevention, endorsed by the Council of the Infectious Diseases Society of America in September 1999. Am. J. Respir. Crit. Care Med. 161:1376-1395. - PubMed
-
- Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. - PubMed
-
- Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. Ottenhoff. 2000. Detection of active tuberculosis infection by T-cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. - PubMed
-
- Arend, S. M., A. Geluk, K. E. van Meijgaarden, J. T. van Dissel, M. Theisen, P. Andersen, and T. H. Ottenhoff. 2000. Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect. Immun. 68:3314-3321. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

