Host MHC class II+ antigen-presenting cells and CD4 cells are required for CD8-mediated graft-versus-leukemia responses following delayed donor leukocyte infusions
- PMID: 16757687
- PMCID: PMC1895539
- DOI: 10.1182/blood-2006-03-007427
Host MHC class II+ antigen-presenting cells and CD4 cells are required for CD8-mediated graft-versus-leukemia responses following delayed donor leukocyte infusions
Abstract
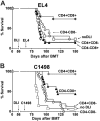
Following bone marrow transplantation, delayed donor leukocyte infusions (DLIs) can induce graft-versus-leukemia (GVL) effects without graft-versus-host disease (GVHD). These antitumor responses are maximized by the presence of host hematopoietic antigen-presenting cells (APCs) at the time of DLI. Using a tumor-protection model, we demonstrate here that GVL activity following administration of DLIs to established mixed chimeras is dependent primarily on reactivity to allogeneic MHC antigens rather than minor histocompatibility or tumor-associated antigens. CD8(+) T-cell-dependent GVL responses against an MHC class II-negative tumor following delayed DLI require CD4(+) T-cell help and are reduced significantly when host APCs lack MHC class II expression. CD4(+) T cells primed by host APCs were required for maximal expansion of graft-versus-host reactive CD8(+) T cells but not their synthesis of IFN-gamma. In contrast, the GVL requirement for CD4(+) T-cell help was bypassed almost completely when DLI was administered to freshly irradiated recipients, indicating that the host environment is a major factor influencing the cellular mechanisms of GVL.
Figures

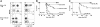

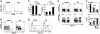
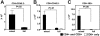

References
-
- Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304: 1529-1533. - PubMed
-
- Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300: 1068-1073. - PubMed
-
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75: 555-562. - PubMed
-
- Mapara MY, Kim YM, Wang SP, Bronson R, Sachs DH, Sykes M. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood. 2002;100: 1903-1909. - PubMed
-
- Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11: 1244-1249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

