Effects of alpha1D-adrenergic receptors on shedding of biologically active EGF in freshly isolated lacrimal gland epithelial cells
- PMID: 16760267
- PMCID: PMC2151204
- DOI: 10.1152/ajpcell.00014.2006
Effects of alpha1D-adrenergic receptors on shedding of biologically active EGF in freshly isolated lacrimal gland epithelial cells
Abstract
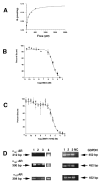
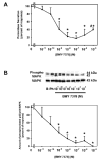
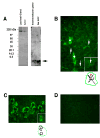
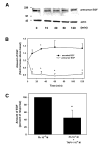
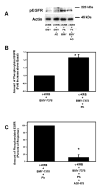
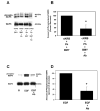
Transactivation of EGF receptors by G protein-coupled receptors is a well-known phenomenon. This process involves the ectodomain shedding of growth factors in the EGF family by matrix metalloproteinases. However, many of these studies employ transformed and/or cultured cells that overexpress labeled growth factors. In addition, few studies have shown that EGF itself is the growth factor that is shed and is responsible for transactivation of the EGF receptor. In this study, we show that freshly isolated, nontransformed lacrimal gland acini express two of the three known alpha(1)-adrenergic receptors (ARs), namely, alpha(1B)- and alpha(1D)-ARs. Alpha(1D)-ARs mediate phenylephrine (an alpha(1)-adrenergic agonist)-induced protein secretion and activation of p42/p44 MAPK, because the alpha(1D)-AR inhibitor BMY-7378, but not the alpha(1A)-AR inhibitor 5-methylurapidil, inhibits these processes. Activation of p42/p44 MAPK occurs through transactivation of the EGF receptor, which is inhibited by the matrix metalloproteinase ADAM17 inhibitor TAPI-1. In addition, phenylephrine caused the shedding of EGF from freshly isolated acini into the buffer. Incubation of freshly isolated cells with conditioned buffer from cells treated with phenylephrine resulted in activation of the EGF receptor and p42/p44 MAPK. The EGF receptor inhibitor AG1478 and an EGF-neutralizing antibody blocked this activation of p42/p44 MAPK. We conclude that in freshly isolated lacrimal gland acini, alpha(1)-adrenergic agonists activate the alpha(1D)-AR to stimulate protein secretion and the ectodomain shedding of EGF to transactivate the EGF receptor, potentially via ADAM17, which activates p42/p44 MAPK to negatively modulate protein secretion.
Figures


Comment in
-
Receptor transactivation cascades. Focus on "Effects of alpha1D-adrenergic receptors on shedding of biologically active EGF in freshly isolated lacrimal gland epithelial cells".Am J Physiol Cell Physiol. 2007 Jan;292(1):C1-3. doi: 10.1152/ajpcell.00364.2006. Epub 2006 Jul 26. Am J Physiol Cell Physiol. 2007. PMID: 16870826 Review. No abstract available.
References
-
- Arevalo-Leon LE, Gallardo-Ortiz IA, Urquiza-Marin H, Villalobos-Molina R. Evidence for the role of alpha1D- and alpha1A-adrenoceptors in contraction of the rat mesenteric artery. Vascul Pharmacol. 2003;40:91–96. - PubMed
-
- Arribas J, Coodly L, Vollmer P, Kishimoto TK, Rose-John S, Massague J. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J Biol Chem. 1996;271:11376–11382. - PubMed
-
- Brown CL, Meise KS, Plowman GD, Coffey RJ, Dempsey PJ. Cell surface ectodomain cleavage of human amphiregulin precursor is sensitive to a metalloprotease inhibitor. Release of a predominant N-glycosylated 43-kDa soluble form. J Biol Chem. 1998;273:17258–17268. - PubMed
-
- Chalothorn D, McCune DF, Edelmann SE, Garcia-Cazarin ML, Tsujimoto G, Piascik MT. Differences in the cellular localization and agonist-mediated internalization properties of the alpha(1)-adrenoceptor subtypes. Mol Pharmacol. 2002;61:1008–1016. - PubMed
-
- Chen J, Acton TB, Basu SK, Montelione GT, Inouye M. Enhancement of the solubility of proteins overexpressed in Escherichia coli by heat shock. J Mol Microbiol Biotechnol. 2002;4:519–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

