Cell wall assembly in Saccharomyces cerevisiae
- PMID: 16760306
- PMCID: PMC1489534
- DOI: 10.1128/MMBR.00038-05
Cell wall assembly in Saccharomyces cerevisiae
Abstract
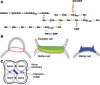
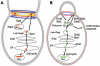
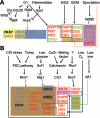
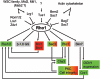
An extracellular matrix composed of a layered meshwork of beta-glucans, chitin, and mannoproteins encapsulates cells of the yeast Saccharomyces cerevisiae. This organelle determines cellular morphology and plays a critical role in maintaining cell integrity during cell growth and division, under stress conditions, upon cell fusion in mating, and in the durable ascospore cell wall. Here we assess recent progress in understanding the molecular biology and biochemistry of cell wall synthesis and its remodeling in S. cerevisiae. We then review the regulatory dynamics of cell wall assembly, an area where functional genomics offers new insights into the integration of cell wall growth and morphogenesis with a polarized secretory system that is under cell cycle and cell type program controls.
Figures

References
-
- Adams, D. J. 2004. Fungal cell wall chitinases and glucanases. Microbiology 150:2029-2035. - PubMed
-
- Agarwal, A. K., P. D. Rogers, S. R. Baerson, M. R. Jacob, K. A. T. H. Barker, J. D. Cleary, L. A. Walker, D. G. Nagle, and A. M. Clark. 2003. Genome-wide expression profiling of the response to polyene, pyrimidine, azole, and echinocandin antifungal agents in Saccharomyces cerevisiae. J. Biol. Chem. 278:34998-35015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

