The continuing story of class IIa bacteriocins
- PMID: 16760314
- PMCID: PMC1489543
- DOI: 10.1128/MMBR.00016-05
The continuing story of class IIa bacteriocins
Abstract
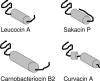
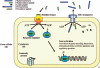
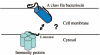
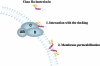
Many bacteria produce antimicrobial peptides, which are also referred to as peptide bacteriocins. The class IIa bacteriocins, often designated pediocin-like bacteriocins, constitute the most dominant group of antimicrobial peptides produced by lactic acid bacteria. The bacteriocins that belong to this class are structurally related and kill target cells by membrane permeabilization. Despite their structural similarity, class IIa bacteriocins display different target cell specificities. In the search for new antibiotic substances, the class IIa bacteriocins have been identified as promising new candidates and have thus received much attention. They kill some pathogenic bacteria (e.g., Listeria) with high efficiency, and they constitute a good model system for structure-function analyses of antimicrobial peptides in general. This review focuses on class IIa bacteriocins, especially on their structure, function, mode of action, biosynthesis, bacteriocin immunity, and current food applications. The genetics and biosynthesis of class IIa bacteriocins are well understood. The bacteriocins are ribosomally synthesized with an N-terminal leader sequence, which is cleaved off upon secretion. After externalization, the class IIa bacteriocins attach to potential target cells and, through electrostatic and hydrophobic interactions, subsequently permeabilize the cell membrane of sensitive cells. Recent observations suggest that a chiral interaction and possibly the presence of a mannose permease protein on the target cell surface are required for a bacteria to be sensitive to class IIa bacteriocins. There is also substantial evidence that the C-terminal half penetrates into the target cell membrane, and it plays an important role in determining the target cell specificity of these bacteriocins. Immunity proteins protect the bacteriocin producer from the bacteriocin it secretes. The three-dimensional structures of two class IIa immunity proteins have been determined, and it has been shown that the C-terminal halves of these cytosolic four-helix bundle proteins specify which class IIa bacteriocin they protect against.
Figures


References
-
- Aasen, I. M., S. Markussen, T. Moretro, T. Katla, L. Axelsson, and K. Naterstad. 2003. Interactions of the bacteriocins sakacin P and nisin with food constituents. Int. J. Food Microbiol. 87:35-43. - PubMed
-
- Axelsson, L., T. Katla, M. Bjørnslett, V. G. H. Eijsink, and A. Holck. 1998. A system for expression of bacteriocins in Lactobacillus sakei. FEMS Microbiol. Lett. 168:137-143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

