Early antibody responses to experimental Mycobacterium bovis infection of cattle
- PMID: 16760322
- PMCID: PMC1489550
- DOI: 10.1128/CVI.00061-06
Early antibody responses to experimental Mycobacterium bovis infection of cattle
Abstract
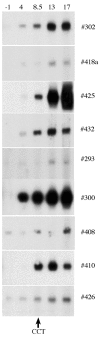
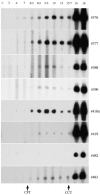
Bovine tuberculosis persists as a costly zoonotic disease in numerous countries despite extensive eradication and control efforts. Sequential serum samples obtained from Mycobacterium bovis-infected cattle were evaluated for seroreactivity to mycobacterial antigens. Animals received M. bovis by aerosol, intratonsil, intranasal, or intratracheal inoculation. Assays included the multiantigen print immunoassay for determination of antigen recognition patterns, immunoblot analysis for sensitive kinetic studies, and the VetTB STAT-PAK test, a novel, rapid test based on lateral-flow technology. Responses to MPB83 were detected for all M. bovis-infected animals regardless of the route or strain of M. bovis used for inoculation. Other less commonly recognized antigens included ESAT-6, CFP-10, and MPB70. Responses to MPB83 were detectable as early as 4 weeks after inoculation, were boosted upon injection of purified protein derivatives for skin testing, and persisted throughout the course of each of the four challenge studies. MPB83-specific immunoglobulin M (IgM) was detected prior to MPB83-specific IgG detection; however, early IgM responses rapidly waned, suggesting a benefit of tests that detect both IgM- and IgG-specific antibodies. The VetTB STAT-PAK test detected responses in sera from 60% (15/25) of the animals by 7 weeks after challenge and detected responses in 96% (24/25) of the animals by 18 weeks. These findings demonstrate the potential for new-generation antibody-based tests for the early detection of M. bovis infection in cattle.
Figures




References
-
- Bannantine, J., and J. R. Stabel. 2000. HspX is present within Mycobacterium paratuberculosis-infected macrophages and is recognized by sera from some infected cattle. Vet. Microbiol. 76:343-358. - PubMed
-
- Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. - PubMed
-
- Corner, L. A., M. A. Stevenson, D. M. Collins, and R. S. Morris. 2003. The re-emergence of Mycobacterium bovis infection in brushtail possums (Trichosurus vulpecula) after localised possum eradication. N. Z. Vet. J. 51(2):73-80. - PubMed
-
- Donnelly, C. A., R. Woodroffe, D. R. Cox, F. J. Bourne, C. L. Cheeseman, R. S. Clifton-Hadley, G. Wei, G. Gettinby, P. Gilks, H. Jenkins, W. T. Johnston, A. M. Le Fevre, J. P. McInerney, and W. I. Morrison. 2005. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature 439:843-846. - PubMed
-
- European Economic Community. 1980. EEC directive 80/219, amending directive 64/432 annexe B. Off. J. L047:25-32.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

