Endocytosis of cadherin from intracellular junctions is the driving force for cadherin adhesive dimer disassembly
- PMID: 16760429
- PMCID: PMC1525252
- DOI: 10.1091/mbc.e06-03-0190
Endocytosis of cadherin from intracellular junctions is the driving force for cadherin adhesive dimer disassembly
Abstract
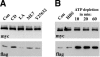
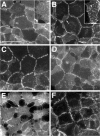
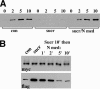
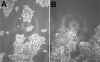
The adhesion receptor E-cadherin maintains cell-cell junctions by continuously forming short-lived adhesive dimers. Here mixed culture cross-linking and coimmunoprecipitation assays were used to determine the dynamics of adhesive dimer assembly. We showed that the amount of these dimers increased dramatically minutes after the inhibition of endocytosis by ATP depletion or by hypertonic sucrose. This increase was accompanied by the efficient recruitment of E-cadherin into adherens junctions. After 10 min, when the adhesive dimer amount had reached a plateau, the assembly of new dimers stalled completely. These cells, in a striking difference from the control, became unable to disintegrate both their intercellular contacts and adhesive dimers in response to calcium depletion. The same effects, but after a slightly longer time course, were obtained using acidic media, another potent approach inhibiting endocytosis. These data suggest that endocytosis is the main pathway for the dissociation of E-cadherin adhesive dimers. Its inhibition blocks the replenishment of the monomeric cadherin pool, thereby inhibiting new dimer formation. This suggestion has been corroborated by immunoelectron microscopy, which revealed cadherin-enriched coated pit-like structures in close association with adherens junctions.
Figures






References
-
- Boggon T. J., Murray J., Chappuis-Flament S., Wong E., Gumbiner B. M., Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. - PubMed
-
- Bryant D. M., Stow J. L. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 2004;14:427–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

