A role for the mitogen-activated protein kinase kinase kinase 1 in epithelial wound healing
- PMID: 16760432
- PMCID: PMC1525243
- DOI: 10.1091/mbc.e06-02-0102
A role for the mitogen-activated protein kinase kinase kinase 1 in epithelial wound healing
Abstract
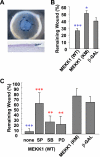
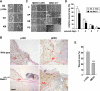
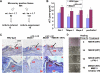
The mitogen-activated protein kinase kinase (MEK) kinase 1 (MEKK1) mediates activin B signals required for eyelid epithelium morphogenesis during mouse fetal development. The present study investigates the role of MEKK1 in epithelial wound healing, another activin-regulated biological process. In a skin wound model, injury markedly stimulates MEKK1 expression and activity, which are in turn required for the expression of genes involved in extracellular matrix (ECM) homeostasis. MEKK1 ablation or down-regulation by interfering RNA significantly delays skin wound closure and impairs activation of Jun NH2-terminal kinases, induction of plasminogen activator inhibitor (PAI)-1, and restoration of cell-cell junctions of the wounded epidermis. Conversely, expression of wild-type MEKK1 accelerates reepithelialization of full-thickness skin and corneal debridement wounds by mechanisms involving epithelial cell migration, a cell function that is partially abolished by neutralizing antibodies for PAI-1 and metalloproteinase III. Our data suggest that MEKK1 transmits wound signals, leading to the transcriptional activation of genes involved in ECM homeostasis, epithelial cell migration, and wound reepithelialization.
Figures


Similar articles
-
MEKK1 transduces activin signals in keratinocytes to induce actin stress fiber formation and migration.Mol Cell Biol. 2005 Jan;25(1):60-5. doi: 10.1128/MCB.25.1.60-65.2005. Mol Cell Biol. 2005. PMID: 15601830 Free PMC article.
-
A role for MEK kinase 1 in TGF-beta/activin-induced epithelium movement and embryonic eyelid closure.EMBO J. 2003 Sep 1;22(17):4443-54. doi: 10.1093/emboj/cdg440. EMBO J. 2003. PMID: 12941696 Free PMC article.
-
JNK MAPK signaling contributes in vivo to injury-induced corneal epithelial migration.Ophthalmic Res. 2009;42(4):185-92. doi: 10.1159/000232401. Epub 2009 Aug 7. Ophthalmic Res. 2009. PMID: 19672126
-
The control of cell motility and epithelial morphogenesis by Jun kinases.Trends Cell Biol. 2004 Feb;14(2):94-101. doi: 10.1016/j.tcb.2003.12.005. Trends Cell Biol. 2004. PMID: 15102441 Review.
-
Roles of P21-activated kinases and associated proteins in epithelial wound healing.Int Rev Cell Mol Biol. 2008;267:253-98. doi: 10.1016/S1937-6448(08)00606-0. Int Rev Cell Mol Biol. 2008. PMID: 18544501 Free PMC article. Review.
Cited by
-
Molecular Targets of Pinocembrin Underlying Its Regenerative Activities in Human Keratinocytes.Pharmaceuticals (Basel). 2022 Jul 31;15(8):954. doi: 10.3390/ph15080954. Pharmaceuticals (Basel). 2022. PMID: 36015102 Free PMC article.
-
Activin B promotes epithelial wound healing in vivo through RhoA-JNK signaling pathway.PLoS One. 2011;6(9):e25143. doi: 10.1371/journal.pone.0025143. Epub 2011 Sep 19. PLoS One. 2011. PMID: 21949871 Free PMC article.
-
JNK Signaling as a Key Modulator of Soft Connective Tissue Physiology, Pathology, and Healing.Int J Mol Sci. 2020 Feb 4;21(3):1015. doi: 10.3390/ijms21031015. Int J Mol Sci. 2020. PMID: 32033060 Free PMC article. Review.
-
Single Dose of N-Acetylcysteine in Local Anesthesia Increases Expression of HIF1α, MAPK1, TGFβ1 and Growth Factors in Rat Wound Healing.Int J Mol Sci. 2021 Aug 12;22(16):8659. doi: 10.3390/ijms22168659. Int J Mol Sci. 2021. PMID: 34445365 Free PMC article.
-
Metalloproteases meprin-ɑ (MEP1A) is a prognostic biomarker and promotes proliferation and invasion of colorectal cancer.BMC Cancer. 2016 Jul 4;16:383. doi: 10.1186/s12885-016-2460-5. BMC Cancer. 2016. PMID: 27378469 Free PMC article.
References
-
- Albo D., Berger D. H., Vogel J., Tuszynski G. P. Thrombospondin-1 and transforming growth factor beta-1 upregulate plasminogen activator inhibitor type 1 in pancreatic cancer. J. Gastrointest. Surg. 1999;3:411–417. - PubMed
-
- Ashcroft G. S., et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1999;1:260–266. - PubMed
-
- Bosch M., Serras F., Martin-Blanco E., Baguna J. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev. Biol. 2005;280:73–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

