Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1
- PMID: 16762632
- PMCID: PMC2648856
- DOI: 10.1053/j.gastro.2006.03.020
Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1
Abstract
Background & aims: The biguanide drug metformin has recently been found to improve steatosis and liver damage in animal models and in humans with nonalcoholic steatohepatitis.
Methods: The aim of the present study was to determine whether metformin also prevents steatosis and liver damage in mouse models of acute and chronic alcohol exposure.
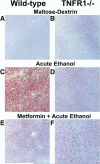
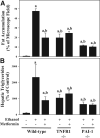
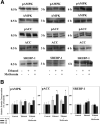
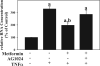
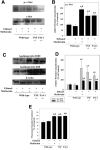
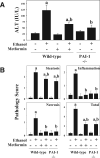
Results: Acute ethanol exposure caused a >20-fold increase in hepatic lipids, peaking 12 hours after administration. Metformin treatment significantly blunted the ethanol effect by >60%. Although metformin is a known inducer of AMP kinase (AMPK) activity, the hepatoprotective property of metformin did not correlate with activation of AMPK or of AMPK-dependent pathways. Instead, the protective effects of metformin correlated with complete prevention of the upregulation of plasminogen activator inhibitor (PAI)-1 caused by ethanol. Indeed, a similar protective effect against acute alcohol-induced lipid accumulation was observed in PAI-1-/- mice. Hepatic fat accumulation caused by chronic enteral ethanol feeding was also prevented by metformin or by knocking out PAI-1. Under these conditions, necroinflammatory changes caused by ethanol were also significantly attenuated.
Conclusions: Taken together, these findings suggest a novel mechanism of action for metformin and identify a new role of PAI-1 in hepatic injury caused by ethanol.
Figures


Comment in
-
Metformin and the fate of fat.Gastroenterology. 2006 Jun;130(7):2234-7. doi: 10.1053/j.gastro.2006.05.009. Gastroenterology. 2006. PMID: 16762646 No abstract available.
References
-
- Grant BF, Dufour MC, Harford TC. Epidemiology of alcoholic liver disease. Semin Liver Dis. 1988;8:12–25. - PubMed
-
- MacSween RN, Burt AD. Histologic spectrum of alcoholic liver disease. Semin Liver Dis. 1986;6:221–232. - PubMed
-
- Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party? Hepatology. 1998;27:1463–1466. - PubMed
-
- Harrison SA, Diehl AM. Fat and the liver—a molecular overview. Semin Gastrointest Dis. 2002;13:3–16. - PubMed
-
- Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

