Neuronal pentraxins mediate synaptic refinement in the developing visual system
- PMID: 16763034
- PMCID: PMC2579897
- DOI: 10.1523/JNEUROSCI.4212-05.2006
Neuronal pentraxins mediate synaptic refinement in the developing visual system
Abstract
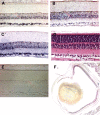
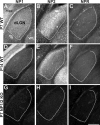
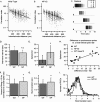
Neuronal pentraxins (NPs) define a family of proteins that are homologous to C-reactive and acute-phase proteins in the immune system and have been hypothesized to be involved in activity-dependent synaptic plasticity. To investigate the role of NPs in vivo, we generated mice that lack one, two, or all three NPs. NP1/2 knock-out mice exhibited defects in the segregation of eye-specific retinal ganglion cell (RGC) projections to the dorsal lateral geniculate nucleus, a process that involves activity-dependent synapse formation and elimination. Retinas from mice lacking NP1 and NP2 had cholinergically driven waves of activity that occurred at a frequency similar to that of wild-type mice, but several other parameters of retinal activity were altered. RGCs cultured from these mice exhibited a significant delay in functional maturation of glutamatergic synapses. Other developmental processes, such as pathfinding of RGCs at the optic chiasm and hippocampal long-term potentiation and long-term depression, appeared normal in NP-deficient mice. These data indicate that NPs are necessary for early synaptic refinements in the mammalian retina and dorsal lateral geniculate nucleus. We speculate that NPs exert their effects through mechanisms that parallel the known role of short pentraxins outside the CNS.
Figures






References
-
- Ball SL, Powers PA, Shin H-S, Morgans CW, Peachey NS, Gregg RG (2002). Role of the β2 subunit of voltage dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci 43:1595–1603. - PubMed
-
- Banker G, Goslin K (1991). In: Culturing nerve cells Cambridge, MA: MIT.
-
- Barres BA, Silverstein BE, Corey DP, Chun LL (1988). Immunological, morphological and electrophysiological variation among retinal ganglion cells purified by panning. Neuron 1:791–803. - PubMed
-
- Boulanger LM, Huh GS, Shatz CJ (2001). Neuronal plasticity and cellular immunity: shared molecular mechanisms. Curr Opin Neurobiol 11:568–578. - PubMed
-
- Breviario F, d'Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, Saccone S, Marzella R, Predazzi V, Rocchi M, Della Valle G, Dejana E, Mantovani A, Introna M (1992). Interleukin-1-inducible genes in endothelial cells: cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem 267:22190–22197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
