Possible origins of the complex topographic organization of motor cortex: reduction of a multidimensional space onto a two-dimensional array
- PMID: 16763036
- PMCID: PMC6675193
- DOI: 10.1523/JNEUROSCI.0768-06.2006
Possible origins of the complex topographic organization of motor cortex: reduction of a multidimensional space onto a two-dimensional array
Abstract
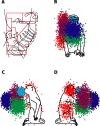
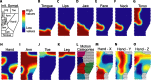
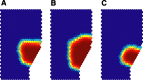
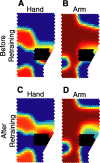
We propose that some of the features of the topographic organization in motor cortex emerge from a competition among several conflicting mapping requisites. These competing requisites include a somatotopic map of the body, a map of hand location in space, and a partitioning of cortex into regions that emphasize different complex, ethologically relevant movements. No one type of map fully explains the topography; instead, all three influences (and perhaps others untested here) interact to form the topography. A standard algorithm (Kohonen network) was used to generate an artificial motor cortex array that optimized local continuity for these conflicting mapping requisites. The resultant hybrid map contained many features seen in actual motor cortex, including the following: a rough, overlapping somatotopy; a posterior strip in which simpler movements were represented and more somatotopic segregation was observed, and an anterior strip in which more complex, multisegmental movements were represented and the somatotopy was less segregated; a clustering of different complex, multisegmental movements into specific subregions of cortex that resembled the arrangement of subregions found in the monkey; three hand representations arranged on the cortex in a manner similar to the primary motor, dorsal premotor, and ventral premotor hand areas in the monkey; and maps of hand location that approximately matched the maps observed in the monkey.
Figures



References
-
- Campbell AW (1905). In: Histological studies on the localization of cerebral function New York: Cambridge UP.
-
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG (1998). Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79:1117–1123. - PubMed
-
- Cooke DF, Graziano MSA (2003). Defensive movements evoked by air puff in monkeys. J Neurophysiol 90:3317–3329. - PubMed
-
- Cooke DF, Graziano MSA (2004a). Sensorimotor integration in the precentral gyrus: polysensory neurons and defensive movements. J Neurophysiol 91:1648–1660. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
