The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity
- PMID: 16763566
- PMCID: PMC1500846
- DOI: 10.1038/sj.emboj.7601166
The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity
Abstract
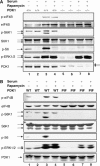
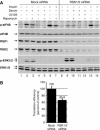
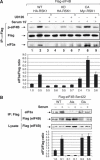
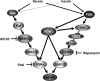
The eukaryotic translation initiation factor 4B (eIF4B) plays a critical role in recruiting the 40S ribosomal subunit to the mRNA. In response to insulin, eIF4B is phosphorylated on Ser422 by S6K in a rapamycin-sensitive manner. Here we demonstrate that the p90 ribosomal protein S6 kinase (RSK) phosphorylates eIF4B on the same residue. The relative contribution of the RSK and S6K modules to the phosphorylation of eIF4B is growth factor-dependent, and the two phosphorylation events exhibit very different kinetics. The S6K and RSK proteins are members of the AGC protein kinase family, and require PDK1 phosphorylation for activation. Consistent with this requirement, phosphorylation of eIF4B Ser422 is abrogated in PDK1 null embryonic stem cells. Phosphorylation of eIF4B on Ser422 by RSK and S6K is physiologically significant, as it increases the interaction of eIF4B with the eukaryotic translation initiation factor 3.
Figures



References
-
- Alessi DR, Kozlowski MT, Weng QP, Morrice N, Avruch J (1998) 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol 8: 69–81 - PubMed
-
- Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S (2005) Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care 8: 67–72 - PubMed
-
- Balendran A, Currie R, Armstrong CG, Avruch J, Alessi DR (1999) Evidence that 3-phosphoinositide-dependent protein kinase-1 mediates phosphorylation of p70 S6 kinase in vivo at Thr-412 as well as Thr-252. J Biol Chem 274: 37400–37406 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

