In vivo delivery of recombinant viruses to the fetal murine cochlea: transduction characteristics and long-term effects on auditory function
- PMID: 16765094
- PMCID: PMC4108343
- DOI: 10.1016/j.ymthe.2006.04.003
In vivo delivery of recombinant viruses to the fetal murine cochlea: transduction characteristics and long-term effects on auditory function
Abstract
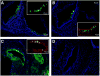
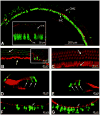
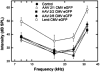
Congenital hearing deficits can be caused by a variety of genetic and acquired conditions. Complete reversal of deficits in the peripheral auditory system may require delivery of corrective genes to cochlear progenitor cells. We tested delivery of lentivirus and an array of recombinant adeno-associated viral (AAV) serotypes for efficiency and cellular specificity of transgene expression after in utero delivery to the developing mouse otocyst. Stability of expression and safety with respect to auditory function were then tested in those vectors that had the most favorable in utero cochlear transduction characteristics (AAV2/1, AAV2/8, and lentivirus). AAV2/1 was found to be the optimal vector for in utero cochlear gene transfer. It efficiently transduced progenitors giving rise to both inner and outer hair cells and supporting cells and had no adverse effect on cochlear cell differentiation. Further, it had no pathological effect on differentiated hair cells or the integrity of the auditory nerve or brain-stem nuclei as measured by auditory brain-stem response testing. AAV2/1 promises to be useful in further studies evaluating differentiation pathways of cochlear cells in health and disease and for developing gene-based therapies for congenital and acquired forms of peripheral hearing loss.
Figures

References
-
- Lalwani A. Development of in vivo gene therapy for hearing disorders: introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther. 1996;3:588–592. - PubMed
-
- Zine A. Molecular mechanisms that regulate auditory hair-cell differentiation in the mammalian cochlea. Mol Neurobiol. 2003;27:223–237. - PubMed
-
- Izumikawa M, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf animals. Nat Med. 2005;11:271–276. - PubMed
-
- Stone I, Lurie D, Kelley M, Poulsen D. Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol Ther. 2005;11:843–848. - PubMed
-
- Han J, et al. Transgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vector. Hum Gene Ther. 1999;10:1867–1873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

