Resonant waveguide grating biosensor for living cell sensing
- PMID: 16766609
- PMCID: PMC1544314
- DOI: 10.1529/biophysj.105.077818
Resonant waveguide grating biosensor for living cell sensing
Abstract
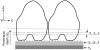
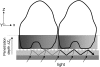
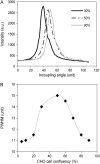
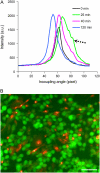
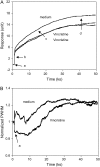
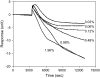
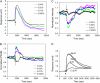
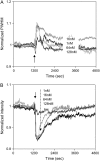
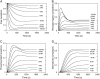
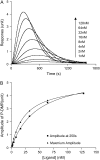
This article presents theoretical analysis and experimental data for the use of resonant waveguide grating (RWG) biosensors to characterize stimulation-mediated cell responses including signaling. The biosensor is capable of detecting redistribution of cellular contents in both directions that are perpendicular and parallel to the sensor surface. This capability relies on online monitoring cell responses with multiple optical output parameters, including the changes in incident angle and the shape of the resonant peaks. Although the changes in peak shape are mainly contributed to stimulation-modulated inhomogeneous redistribution of cellular contents parallel to the sensor surface, the shift in incident angle primarily reflects the stimulation-triggered dynamic mass redistribution (DMR) perpendicular to the sensor surface. The optical signatures are obtained and used to characterize several cellular processes including cell adhesion and spreading, detachment and signaling by trypsinization, and signaling through either epidermal growth factor receptor or bradykinin B2 receptor. A mathematical model is developed to link the bradykinin-mediated DMR signals to the dynamic relocation of intracellular proteins and the receptor internalization during B2 receptor signaling cycle. This model takes the form of a set of nonlinear, ordinary differential equations that describe the changes in four different states of B2 receptors, diffusion of proteins and receptor-protein complexes, and the DMR responses. Classical analysis shows that the system converges to a unique optical signature, whose dynamics (amplitudes, transition time, and kinetics) is dependent on the bradykinin signal input, and consistent with those observed using the RWG biosensors. This study provides fundamentals for probing living cells with the RWG biosensors, in general, optical biosensors.
Figures


References
-
- Blake, R. A. 2001. Cellular screening assays using fluorescence microscopy. Curr. Opin. Pharmacol. 1:533–538. - PubMed
-
- Taylor, D. L., E. S. Woo, and K. A. Giuliano. 2001. Real-time molecular and cellular analysis: the new frontier of drug discovery. Curr. Opin. Biotechnol. 12:75–81. - PubMed
-
- Abraham, V., D. L. Taylor, and J. R. Haskins. 2004. High content screening applied to large-scale cell biology. Trends Biotechnol. 22:15–22. - PubMed
-
- Gribbon, P., and A. Sewing. 2003. Fluorescence readouts in HTS: no gain without pain? Drug Discov. Today. 8:1035–1043. - PubMed
-
- Balla, T., and P. Varnai. 2002. Visualizing cellular phosphoinositide pools with GFP-fused protein-modules. Sci. STKE. 125:pI3. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

