Biosynthesis of ascorbic acid in legume root nodules
- PMID: 16766673
- PMCID: PMC1489911
- DOI: 10.1104/pp.106.081463
Biosynthesis of ascorbic acid in legume root nodules
Abstract
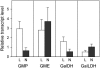
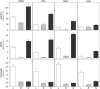
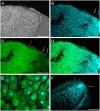
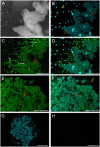
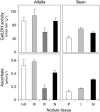
Ascorbic acid (vitamin C) is a major antioxidant and redox buffer, but is also involved in other critical processes of plants. Recently, the hypothesis has been proposed that legume nodules are unable to synthesize ascorbate and have to import it from the shoot or root, thus providing a means by which the plant regulates nodule senescence. The last step of ascorbate biosynthesis in plants is catalyzed by L-galactono-1,4-lactone dehydrogenase (GalLDH). The mRNAs encoding GalLDH and three other enzymes involved in ascorbate biosynthesis are clearly detectable in nodules. Furthermore, an active membrane-bound GalLDH enzyme is present in nodule mitochondria. Biochemical assays on dissected nodules reveal that GalLDH activity and ascorbate are correlated in nodule tissues and predominantly localized in the infected zone, with lower levels of both parameters (relative to the infected tissues) in the apex (87%) and senescent region (43%) of indeterminate nodules and in the peripheral tissues (65%) of determinate nodules. In situ RNA hybridization showed that the GalLDH mRNA is particularly abundant in the infected zone of indeterminate and determinate nodules. Thus, our results refute the hypothesis that ascorbate is not synthesized in nodules and lend support to a previous conclusion that ascorbate in the infected zone is primarily involved in the protection of host cells against peroxide damage. Likewise, the high ascorbate and GalLDH activity levels found in the apex of indeterminate nodules strongly suggest a participation of ascorbate in additional functions during symbiosis, possibly related to cell growth and division and to molecular signaling.
Figures

References
-
- Alesandrini F, Mathis R, Van de Sype G, Hérouart D, Puppo A (2003) Possible roles for a cysteine protease and hydrogen peroxide in soybean nodule development and senescence. New Phytol 158: 131–138
-
- Arrigoni O, Arrigoni-Liso R, Calabrese G (1977) Ascorbic acid requirement for biosynthesis of hydroxyproline-containing proteins in plants. FEBS Lett 82: 135–138 - PubMed
-
- Arrigoni O, De Tullio MC (2002) Ascorbic acid: much more than an antioxidant. Biochim Biophys Acta 1569: 1–9 - PubMed
-
- Bartoli CG, Guiamet JJ, Kiddle G, Pastori GM, Di Cagno R, Theodoulou FL, Foyer CH (2005) Ascorbate content of wheat leaves is not determined by maximal L-galactono-1,4-lactone dehydrogenase (GalLDH) activity under drought stress. Plant Cell Environ 28: 1073–1081
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

