Identification of features regulating OST1 kinase activity and OST1 function in guard cells
- PMID: 16766677
- PMCID: PMC1533939
- DOI: 10.1104/pp.106.079327
Identification of features regulating OST1 kinase activity and OST1 function in guard cells
Abstract
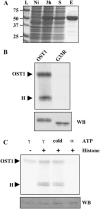
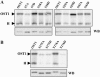
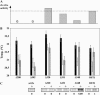
The phytohormone abscisic acid (ABA) mediates drought responses in plants and, in particular, triggers stomatal closure. Snf1-related kinase 2 (SnRK2) proteins from several plant species have been implicated in ABA-signaling pathways. In Arabidopsis (Arabidopsis thaliana) guard cells, OPEN STOMATA 1 (OST1)/SRK2E/SnRK2-6 is a critical positive regulator of ABA signal transduction. A better understanding of the mechanisms responsible for SnRK2 protein kinase activation is thus a major goal toward understanding ABA signal transduction. Here, we report successful purification of OST1 produced in Escherichia coli: The protein is active and autophosphorylates. Using mass spectrometry, we identified five target residues of autophosphorylation in recombinant OST1. Sequence analysis delineates two conserved boxes located in the carboxy-terminal moiety of OST1 after the catalytic domain: the SnRK2-specific box (glutamine-303 to proline-318) and the ABA-specific box (leucine-333 to methionine-362). Site-directed mutagenesis and serial deletions reveal that serine (Ser)-175 in the activation loop and the SnRK2-specific box are critical for the activity of recombinant OST1 kinase. Targeted expression of variants of OST1 kinase in guard cells uncovered additional features that are critical for OST1 function in ABA signaling, although not required for OST1 kinase activity: Ser-7, Ser-18, and Ser-29 and the ABA-specific box. Ser-7, Ser-18, Ser-29, and Ser-43 represent putative targets for regulatory phosphorylation and the ABA-specific box may be a target for the binding of signaling partners in guard cells.
Figures







References
-
- Adams JA (2003) Activation loop phosphorylation and catalysis in protein kinases: Is there functional evidence for the autoinhibitor model? Biochemistry 42: 601–607 - PubMed
-
- Bensmihen S, To A, Lambert G, Kroj T, Giraudat J, Parcy F (2004) Analysis of an activated ABI5 allele using a new selection method for transgenic Arabidopsis seeds. FEBS Lett 561: 127–131 - PubMed
-
- Boudsocq M, Barbier-Brygoo H, Lauriere C (2004) Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J Biol Chem 279: 41758–41766 - PubMed
-
- Droillard MJ, Thibivilliers S, Cazale AC, Barbier-Brygoo H, Lauriere C (2000) Protein kinases induced by osmotic stresses and elicitor molecules in tobacco cell suspensions: two crossroad MAP kinases and one osmoregulation-specific protein kinase. FEBS Lett 474: 217–222 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

