CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy
- PMID: 16767219
- PMCID: PMC1474817
- DOI: 10.1172/JCI27438
CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy
Abstract
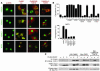
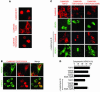
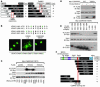
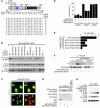
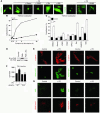
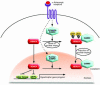
Class IIa histone deacetylases (HDACs) regulate a variety of cellular processes, including cardiac growth, bone development, and specification of skeletal muscle fiber type. Multiple serine/threonine kinases control the subcellular localization of these HDACs by phosphorylation of common serine residues, but whether certain class IIa HDACs respond selectively to specific kinases has not been determined. Here we show that calcium/calmodulin-dependent kinase II (CaMKII) signals specifically to HDAC4 by binding to a unique docking site that is absent in other class IIa HDACs. Phosphorylation of HDAC4 by CaMKII promotes nuclear export and prevents nuclear import of HDAC4, with consequent derepression of HDAC target genes. In cardiomyocytes, CaMKII phosphorylation of HDAC4 results in hypertrophic growth, which can be blocked by a signal-resistant HDAC4 mutant. These findings reveal a central role for HDAC4 in CaMKII signaling pathways and have implications for the control of gene expression by calcium signaling in a variety of cell types.
Figures

References
-
- Roth S.Y., Denu J.M., Allis C.D. Histone acetyltransferases. Annu. Rev. Biochem. 2001;70:81–120. - PubMed
-
- Grozinger C.M., Schreiber S.L. Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem. Biol. 2002;9:3–16. - PubMed
-
- Backs J., Olson E.N. Control of cardiac growth by histone acetylation/deacetylation. Circ. Res. 2006;98:15–24. - PubMed
-
- Verdin E., Dequiedt F., Kasler H.G. Class II histone deacetylases: versatile regulators. Trends Genet. 2003;19:286–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

