Glucose deprivation activates diversity of potassium channels in cultured rat hippocampal neurons
- PMID: 16767515
- PMCID: PMC11520758
- DOI: 10.1007/s10571-006-9000-9
Glucose deprivation activates diversity of potassium channels in cultured rat hippocampal neurons
Abstract
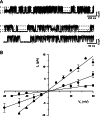
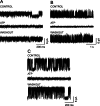
1. Glucose is one of the most important substrates for generating metabolic energy required for the maintenance of cellular functions. Glucose-mediated changes in neuronal firing pattern have been observed in the central nervous system of mammals. K(+) channels directly regulated by intracellular ATP have been postulated as a linkage between cellular energetic metabolism and excitability; the functional roles ascribed to these channels include glucose-sensing to regulate energy homeostasis and neuroprotection under energy depletion conditions. The hippocampus is highly sensitive to metabolic insults and is the brain region most sensitive to ischemic damage. Because the identity of metabolically regulated potassium channels present in hippocampal neurons is obscure, we decided to study the biophysical properties of glucose-sensitive potassium channels in hippocampal neurons. 2. The dependence of membrane potential and the sensitivity of potassium channels to glucose and ATP in rat hippocampal neurons were studied in cell-attached and excised inside-out membrane patches. 3. We found that under hypoglycemic conditions, at least three types of potassium channels were activated; their unitary conductance values were 37, 147, and 241 pS in symmetrical K(+), and they were sensitive to ATP. For K(+) channels with unitary conductance of 37 and 241, when the membrane potential was depolarized the longer closed time constant diminished and this produced an increase in the open-state probability; nevertheless, the 147-pS channels were not voltage-dependent. 4. We propose that neuronal glucose-sensitive K(+) channels in rat hippocampus include subtypes of ATP-sensitive channels with a potential role in neuroprotection during short-term or prolonged metabolic stress.
Figures


Similar articles
-
Glucose and hippocampal neuronal excitability: role of ATP-sensitive potassium channels.J Neurosci Res. 2007 May 15;85(7):1468-77. doi: 10.1002/jnr.21284. J Neurosci Res. 2007. PMID: 17410601
-
Analysis of single K(ATP) channels in mammalian dentate gyrus granule cells.J Neurophysiol. 2000 Nov;84(5):2291-301. doi: 10.1152/jn.2000.84.5.2291. J Neurophysiol. 2000. PMID: 11067973
-
Modulation of K+ channels by intracellular ATP in human neocortical neurons.J Neurophysiol. 1997 Jan;77(1):93-102. doi: 10.1152/jn.1997.77.1.93. J Neurophysiol. 1997. PMID: 9120601
-
Hippocampal hypoglycaemia-activated K+ channels: single-channel analysis of glucose and voltage dependence.Pflugers Arch. 1994 Nov;429(1):58-63. doi: 10.1007/BF02584030. Pflugers Arch. 1994. PMID: 7708482
-
Dissociation between sensing and metabolism of glucose in sugar sensing neurones.J Physiol. 2009 Jan 15;587(1):41-8. doi: 10.1113/jphysiol.2008.163410. Epub 2008 Nov 3. J Physiol. 2009. PMID: 18981030 Free PMC article. Review.
Cited by
-
Rapid activity-dependent modulation of the intrinsic excitability through up-regulation of KCNQ/Kv7 channel function in neonatal spinal motoneurons.PLoS One. 2018 Mar 26;13(3):e0193948. doi: 10.1371/journal.pone.0193948. eCollection 2018. PLoS One. 2018. PMID: 29579068 Free PMC article.
-
A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome.Neuron. 2010 Jun 10;66(5):671-80. doi: 10.1016/j.neuron.2010.04.030. Neuron. 2010. PMID: 20547126 Free PMC article.
References
-
- Aguilar-Bryan, L., and Bryan, J. (1999). Molecular biology of adenosine triphosphate-senstive potassium channels. Endocr. Rev.20:101–135. - PubMed
-
- Aguilar-Bryan, L., Clement, J. P. T., Gonzalez, G., Kunjilwar, K., Babenko, A., and Bryan, J. (1998). Toward understanding the assembly and structure of KATP channels. Physiol. Rev.78:227–245. - PubMed
-
- Ashcroft, F. M. (1988). Adenosine 5′-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci.11:97–118. - PubMed
-
- Ashcroft, F. M., and Gribble, F. M. (1998). Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci.21(7):288–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

