Embryonic pig pancreatic tissue transplantation for the treatment of diabetes
- PMID: 16768546
- PMCID: PMC1479387
- DOI: 10.1371/journal.pmed.0030215
Embryonic pig pancreatic tissue transplantation for the treatment of diabetes
Abstract
Background: Transplantation of embryonic pig pancreatic tissue as a source of insulin has been suggested for the cure of diabetes. However, previous limited clinical trials failed in their attempts to treat diabetic patients by transplantation of advanced gestational age porcine embryonic pancreas. In the present study we examined growth potential, functionality, and immunogenicity of pig embryonic pancreatic tissue harvested at different gestational ages.
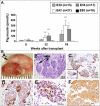
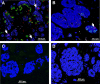
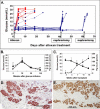
Methods and findings: Implantation of embryonic pig pancreatic tissues of different gestational ages in SCID mice reveals that embryonic day 42 (E42) pig pancreas can enable a massive growth of pig islets for prolonged periods and restore normoglycemia in diabetic mice. Furthermore, both direct and indirect T cell rejection responses to the xenogeneic tissue demonstrated that E42 tissue, in comparison to E56 or later embryonic tissues, exhibits markedly reduced immunogenicity. Finally, fully immunocompetent diabetic mice grafted with the E42 pig pancreatic tissue and treated with an immunosuppression protocol comprising CTLA4-Ig and anti-CD40 ligand (anti-CD40L) attained normal blood glucose levels, eliminating the need for insulin.
Conclusions: These results emphasize the importance of selecting embryonic tissue of the correct gestational age for optimal growth and function and for reduced immunogenicity, and provide a proof of principle for the therapeutic potential of E42 embryonic pig pancreatic tissue transplantation in diabetes.
Conflict of interest statement
Figures

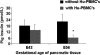

Similar articles
-
Pig embryonic pancreatic tissue as a source for transplantation in diabetes: transient treatment with anti-LFA1, anti-CD48, and FTY720 enables long-term graft maintenance in mice with only mild ongoing immunosuppression.Diabetes. 2009 Jul;58(7):1585-94. doi: 10.2337/db09-0112. Epub 2009 Apr 28. Diabetes. 2009. PMID: 19401429 Free PMC article.
-
Co-stimulatory molecules in islet xenotransplantation: CTLA4Ig treatment in CD40 ligand-deficient mice.Cell Transplant. 2002;11(7):715-20. doi: 10.3727/000000002783985440. Cell Transplant. 2002. PMID: 12518898
-
Fetal pig pancreas. Preparation and assessment of tissue for transplantation, and its in vivo development and function in athymic (nude) mice.Transplantation. 1990 Mar;49(3):571-81. doi: 10.1097/00007890-199003000-00019. Transplantation. 1990. PMID: 1969185
-
Islet xenotransplantation: what is the optimal age of the islet-source pig?Xenotransplantation. 2015 Jan-Feb;22(1):7-19. doi: 10.1111/xen.12130. Epub 2014 Aug 11. Xenotransplantation. 2015. PMID: 25130196 Review.
-
Pancreas transplantation: is islet transplantation the future?Transplant Proc. 1992 Dec;24(6):2379-82. Transplant Proc. 1992. PMID: 1465802 Review. No abstract available.
Cited by
-
From Beta cell replacement to beta cell regeneration: implications for antidiabetic therapy.J Diabetes Sci Technol. 2014 Nov;8(6):1221-6. doi: 10.1177/1932296814540611. Epub 2014 Jun 22. J Diabetes Sci Technol. 2014. PMID: 25355714 Free PMC article. Review.
-
Subcutaneous transplantation of embryonic pancreas for correction of type 1 diabetes.Am J Physiol Endocrinol Metab. 2009 Feb;296(2):E323-32. doi: 10.1152/ajpendo.90544.2008. Epub 2008 Dec 9. Am J Physiol Endocrinol Metab. 2009. PMID: 19066321 Free PMC article.
-
Normalization of glucose post-transplantation into diabetic rats of pig pancreatic primordia preserved in vitro.Organogenesis. 2008 Jan;4(1):48-51. doi: 10.4161/org.5747. Organogenesis. 2008. PMID: 19279715 Free PMC article.
-
Therapeutic window, a critical developmental stage for stem cell therapies.Curr Stem Cell Res Ther. 2010 Dec;5(4):297-3. Curr Stem Cell Res Ther. 2010. PMID: 20528752 Free PMC article. Review.
-
Xenotransplantation of embryonic pig kidney or pancreas to replace the function of mature organs.J Transplant. 2011;2011:501749. doi: 10.1155/2011/501749. Epub 2010 Dec 28. J Transplant. 2011. PMID: 21234246 Free PMC article.
References
-
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. - PubMed
-
- Dunstan DW, Zimmet PZ, Welborn TA, De Courten MP, Cameron AJ, et al. The rising prevalence of diabetes and impaired glucose tolerance: The Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2002;25:829–834. - PubMed
-
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. - PubMed
-
- White NH, Cleary PA, Dahms W, Goldstein D, Malone J, et al. Beneficial effects of intensive therapy of diabetes during adolescence. J Pediatr. 2001;139:804–812. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

