Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary
- PMID: 16768790
- PMCID: PMC1526757
- DOI: 10.1186/1741-7007-4-17
Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary
Abstract
Background: The processes by which eggs develop in the insect ovary are well characterized. Despite a large number of Drosophila mutants that cannot lay eggs, the way that the egg is moved along the reproductive tract from ovary to uterus is less well understood. We remedy this with an integrative study on the reproductive tract muscles (anatomy, innervation, contractions, aminergic modulation) in female flies.
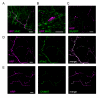
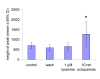
Results: Each ovary, consisting of 15-20 ovarioles, is surrounded by a contractile meshwork, the peritoneal sheath. Individual ovarioles are contained within a contractile epithelial sheath. Both sheaths contain striated muscle fibres. The oviduct and uterine walls contain a circular striated muscle layer. No longitudinal muscle fibres are seen. Neurons that innervate the peritoneal sheath and lateral oviduct have many varicosities and terminate in swellings just outside the muscles of the peritoneal sheath. They all express tyrosine decarboxylase (required for tyramine and octopamine synthesis) and Drosophila vesicular monoamine transporter (DVMAT). No fibres innervate the ovarioles. The common oviduct and uterus are innervated by two classes of neurons, one with similar morphology to those of the peritoneal sheath and another with repeated branches and axon endings similar to type I neuromuscular junctions. In isolated genital tracts from 3- and 7-day old flies, each ovariole contracts irregularly (12.5 +/- 6.4 contractions/minute; mean +/- 95% confidence interval). Peritoneal sheath contractions (5.7 +/- 1.6 contractions/minute) move over the ovary, from tip to base or vice versa, propagating down the oviduct. Rhythmical spermathecal rotations (1.5 +/- 0.29 contractions/minute) also occur. Each genital tract organ exhibits its own endogenous myogenic rhythm. The amplitude of contractions of the peritoneal sheath increase in octopamine (100 nM, 81% P < 0.02) but 1 microM tyramine has no effect. Neither affects the frequency of peritoneal sheath contractions.
Conclusion: The muscle fibres of the reproductive tract are circular and have complex bursting myogenic rhythms under octopaminergic neuromodulation. We propose a new model of tissue-specific actions of octopamine, in which strengthening of peritoneal sheath contractions, coupled with relaxation of the oviduct, eases ovulation. This model accounts for reduced ovulation in flies with mutations in the octopaminergic system.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

