Antilymphoid antibody preconditioning and tacrolimus monotherapy for pediatric kidney transplantation
- PMID: 16769394
- PMCID: PMC2955284
- DOI: 10.1016/j.jpeds.2006.01.008
Antilymphoid antibody preconditioning and tacrolimus monotherapy for pediatric kidney transplantation
Abstract
Objective: Heavy post-transplant immunosuppression may contribute to long-term immunosuppression dependence by subverting tolerogenic mechanisms; thus, we sought to determine if this undesirable consequence could be mitigated by pretransplant lymphoid depletion and minimalistic post-transplant monotherapy.
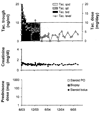
Study design: Lymphoid depletion in 17 unselected pediatric recipients of live (n = 14) or deceased donor kidneys (n = 3) was accomplished with antithymocyte globulin (ATG) (n = 8) or alemtuzumab (n = 9). Tacrolimus was begun post-transplantation with subsequent lengthening of intervals between doses (spaced weaning). Maintenance immunosuppression, morbidity, graft function, and patient/graft survival were collated.
Results: Steroids were added temporarily to treat rejection in two patients (both ATG subgroup) or to treat hemolytic anemia in two others. After 16 to 31 months (mean 22), patient and graft survival was 100% and 94%, respectively. The only graft loss was in a nonweaned noncompliant recipient. In the other 16, serum creatinine was 0.85 +/- 0.35 mg/dL and creatinine clearance was 90.8 +/- 22.1 mL/1.73 m2. All 16 patients are on monotherapy (15 tacrolimus, one sirolimus), and 14 receive every other day or 3 times per week doses. There were no wound or other infections. Two patients developed insulin-dependent diabetes.
Conclusion: The strategy of lymphoid depletion and minimum post-transplant immunosuppression appears safe and effective for pediatric kidney recipients.
Figures


References
-
- Starzl TE. Experience in Renal Transplantation. Philadelphia: WB Saunders Company; 1964. pp. 1–383.
-
- Williams GM, Lee HM, Hume DM. Renal transplants in children. Transplant Proc. 1969;1:262–266. - PubMed
-
- Fine RN, Korsch BM, Stiles Q, Riddell H, Edelbrock HH, Brennan LP, et al. Renal homotransplantation in children. J Pediatr. 1970;76:347–357. - PubMed
-
- Riley CM. Thoughts about kidney homotransplantations in children. J. Pediatr. 1964;65:797. [Editorial]
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

