Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial
- PMID: 16769748
- PMCID: PMC1482338
- DOI: 10.1136/bmj.38867.631551.55
Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial
Abstract
Objective: To compare the effectiveness of clomifene citrate plus metformin and clomifene citrate plus placebo in women with newly diagnosed polycystic ovary syndrome.
Design: Randomised clinical trial.
Setting: Multicentre trial in 20 Dutch hospitals.
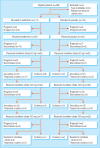
Participants: 228 women with polycystic ovary syndrome.
Interventions: Clomifene citrate plus metformin or clomifene citrate plus placebo.
Main outcome measure: The primary outcome measure was ovulation. Secondary outcome measures were ongoing pregnancy, spontaneous abortion, and clomifene resistance.
Results: 111 women were allocated to clomifene citrate plus metformin (metformin group) and 114 women were allocated to clomifene citrate plus placebo (placebo group). The ovulation rate in the metformin group was 64% compared with 72% in the placebo group, a non-significant difference (risk difference - 8%, 95% confidence interval - 20% to 4%). There were no significant differences in either rate of ongoing pregnancy (40% v 46%; - 6%, - 20% to 7%) or rate of spontaneous abortion (12% v 11%; 1%, - 7% to 10%). A significantly larger proportion of women in the metformin group discontinued treatment because of side effects (16% v 5%; 11%, 5% to 16%).
Conclusion: Metformin is not an effective addition to clomifene citrate as the primary method of inducing ovulation in women with polycystic ovary syndrome.
Trial registration: Current Controlled Trials ISRCTN55906981 [controlled-trials.com].
Figures
Comment in
-
Drugs for anovulatory infertility in polycystic ovary syndrome.BMJ. 2006 Jun 24;332(7556):1461-2. doi: 10.1136/bmj.332.7556.1461. BMJ. 2006. PMID: 16793784 Free PMC article. No abstract available.
References
-
- Fauser BC, The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19: 1-7. - PubMed
-
- Franks S. Polycystic ovary syndrome. N Engl J Med 1995;333: 853-61. - PubMed
-
- Homburg R. What is polycystic ovarian syndrome? A proposal for a consensus on the definition and diagnosis of polycystic ovarian syndrome. Hum Reprod 2002;17: 2495-9. - PubMed
-
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;38: 1165-74. - PubMed
-
- O'Meara NM, Blackman JD, Ehrmann DA, Barnes RB, Jaspan JB, Rosenfield RL, et al. Defects in beta-cell function in functional ovarian hyperandrogenism. J Clin Endocrinol Metab 1993;76: 1241-7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
