The changes in Ca2+ sparks associated with measured modifications of intra-store Ca2+ concentration in skeletal muscle
- PMID: 16769796
- PMCID: PMC2151548
- DOI: 10.1085/jgp.200609545
The changes in Ca2+ sparks associated with measured modifications of intra-store Ca2+ concentration in skeletal muscle
Abstract
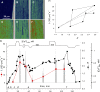
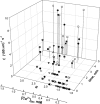
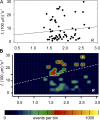
In cardiac muscle and amphibian skeletal muscle, the intracellular Ca2+ release that signals contractile activation proceeds by discrete local packets, which result in Ca2+ sparks. The remarkably stereotyped duration of these release events requires a robustly timed termination mechanism. In cardiac muscle the mechanism of spark termination appears to crucially involve depletion of Ca2+ in the lumen of the sarcoplasmic reticulum (SR), but in skeletal muscle, the mechanism is unknown. We used SEER (shifted excitation and emission ratioing of fluorescence) of SR-trapped mag-indo-1 and confocal imaging of fluorescence of cytosolic rhod-2 to image Ca2+ sparks while reversibly changing and measuring [Ca2+] in the SR ([Ca2+]SR) of membrane-permeabilized frog skeletal muscle cells. Sparks were collected in cells immersed in a solution promoting production of events at moderate frequency. Just after permeabilization, event frequency was zero, and in 10 minutes it reached close to a steady value. Controlled interventions modified [Ca2+]SR reversibly between a low value (299 microM on average in 10 experiments) and a high value (433 microM, a 45% average increase). This change increased sparks frequency by 93%, spatial width by 7%, rise time by 10%, and peak amplitude by 38% (provided that it was calculated in absolute terms, rather than normalized by resting fluorescence). The changes in event frequency and amplitude were statistically significant. The "strength" of the effect of [Ca2+]SR on frequency, quantified by decomposition of variance, was <6%. While the average change in [Ca2+]SR was limited, it reached up to 200% in individual fibers, without causing massive Ca2+ release or an increase of >3.5-fold in event frequency. Taken together with existing evidence that depletion is modest during Ca2+ sparks or release elicited by an action potential, the mild effects of [Ca2+]SR reported here do not support a major role of depletion in either the termination of sparks or the strong inactivation that terminates Ca2+ release at the global level in frog skeletal muscle.
Figures



References
-
- Cramér, H. 1946. Mathematical Methods of Statistics. Princeton University Press, Princeton, NJ. 467.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

