TIP47 is a key effector for Rab9 localization
- PMID: 16769818
- PMCID: PMC2063917
- DOI: 10.1083/jcb.200510010
TIP47 is a key effector for Rab9 localization
Abstract
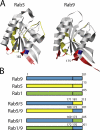
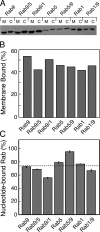
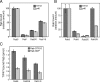
The human genome encodes approximately 70 Rab GTPases that localize to the surfaces of distinct membrane compartments. To investigate the mechanism of Rab localization, chimeras containing heterologous Rab hypervariable domains were generated, and their ability to bind seven Rab effectors was quantified. Two chimeras could bind effectors for two distinctly localized Rabs; a Rab5/9 hybrid bound both Rab5 and Rab9 effectors, and a Rab1/9 hybrid bound to certain Rab1 and Rab9 effectors. These unusual chimeras permitted a test of the importance of effector binding for Rab localization. In both cases, changing the cellular concentration of a key Rab9 effector, which is called tail-interacting protein of 47 kD, moved a fraction of the proteins from their parental Rab localization to that of Rab9. Thus, relative concentrations of certain competing effectors could determine a chimera's localization. These data confirm the importance of effector interactions for Rab9 localization, and support a model in which effector proteins rely on Rabs as much as Rabs rely on effectors to achieve their correct steady state localizations.
Figures
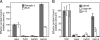
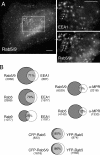
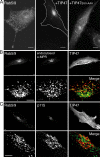
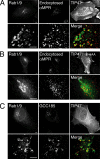
References
-
- Ali, B.R., C. Wasmeier, L. Lamoreux, M. Strom, and M.C. Seabra. 2004. Multiple regions contribute to membrane targeting of Rab GTPases. J. Cell Sci. 117:6401–6412. - PubMed
-
- Allan, B.B., B.D. Moyer, and W.E. Balch. 2000. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 289:444–448. - PubMed
-
- Brennwald, P., and P. Novick. 1993. Interactions of three domains distinguishing the Ras-related GTP-binding proteins Ypt1 and Sec4. Nature. 362:560–563. - PubMed
-
- Burguete, A.S., P.B. Harbury, and S.R. Pfeffer. 2004. In vitro selection and successful prediction of TIP47 protein-interaction interfaces. Nat. Methods. 1:55–60. - PubMed
-
- Carroll, K.S., J. Hanna, I. Simon, J. Krise, P. Barbero, and S.R. Pfeffer. 2001. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science. 292:1373–1376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

