The Sec1p/Munc18 protein Vps45p binds its cognate SNARE proteins via two distinct modes
- PMID: 16769821
- PMCID: PMC3215948
- DOI: 10.1083/jcb.200512024
The Sec1p/Munc18 protein Vps45p binds its cognate SNARE proteins via two distinct modes
Abstract
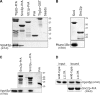
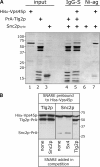
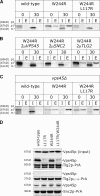
Sec1p/Munc18 (SM) proteins are essential for SNARE-mediated membrane trafficking. The formulation of unifying hypotheses for the function of the SM protein family has been hampered by the observation that two of its members bind their cognate syntaxins (Sxs) in strikingly different ways. The SM protein Vps45p binds its Sx Tlg2p in a manner analogous to that captured by the Sly1p-Sed5p crystal structure, whereby the NH2-terminal peptide of the Sx inserts into a hydrophobic pocket on the outer face of domain I of the SM protein. In this study, we report that although this mode of interaction is critical for the binding of Vps45p to Tlg2p, the SM protein also binds Tlg2p-containing SNARE complexes via a second mode that involves neither the NH2 terminus of Tlg2p nor the region of Vps45p that facilitates this interaction. Our findings point to the possibility that SM proteins interact with their cognate SNARE proteins through distinct mechanisms at different stages in the SNARE assembly/disassembly cycle.
Figures



References
-
- Bracher, A., A. Perrakis, T. Dresbach, H. Betz, and W. Weissenhorn. 2000. The X-ray crystal structure of neuronal Sec1 from squid sheds new light on the role of this protein in exocytosis. Structure. 8:685–894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

