Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle
- PMID: 16769824
- PMCID: PMC2063916
- DOI: 10.1083/jcb.200603119
Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle
Abstract
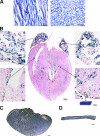
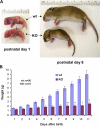
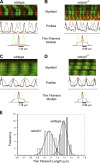
Nebulin is a giant modular sarcomeric protein that has been proposed to play critical roles in myofibrillogenesis, thin filament length regulation, and muscle contraction. To investigate the functional role of nebulin in vivo, we generated nebulin-deficient mice by using a Cre knock-in strategy. Lineage studies utilizing this mouse model demonstrated that nebulin is expressed uniformly in all skeletal muscles. Nebulin-deficient mice die within 8-11 d after birth, with symptoms including decreased milk intake and muscle weakness. Although myofibrillogenesis had occurred, skeletal muscle thin filament lengths were up to 25% shorter compared with wild type, and thin filaments were uniform in length both within and between muscle types. Ultrastructural studies also demonstrated a critical role for nebulin in the maintenance of sarcomeric structure in skeletal muscle. The functional importance of nebulin in skeletal muscle function was revealed by isometric contractility assays, which demonstrated a dramatic reduction in force production in nebulin-deficient skeletal muscle.
Figures





References
-
- Agbulut, O., P. Noirez, F. Beaumont, and G. Butler-Browne. 2003. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol. Cell. 95:399–406. - PubMed
-
- Bang, M.L., C. Gregorio, and S. Labeit. 2002. Molecular dissection of the interaction of desmin with the C-terminal region of nebulin. J. Struct. Biol. 137:119–127. - PubMed
-
- Burkholder, T.J., B. Fingado, S. Baron, and R.L. Lieber. 1994. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hind limb. J. Morphol. 221:177–190. - PubMed
-
- Chen, J., S.W. Kubalak, S. Minamisawa, R.L. Price, K.D. Becker, R. Hickey, J. Ross Jr., and K.R. Chien. 1998. Selective requirement of myosin light chain 2v in embryonic heart function. J. Biol. Chem. 273:1252–1256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

