The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases
- PMID: 16769891
- PMCID: PMC1480460
- DOI: 10.1073/pnas.0600979103
The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases
Abstract
Platelet-derived lysophosphatidic acid (LPA) supports the progression of breast and ovarian cancer metastasis to bone. The mechanisms through which LPA promotes bone metastasis formation are, however, unknown. Here we report that silencing of the type 1 LPA receptor (LPA(1)) in cancer cells blocks the production of tumor-derived cytokines that are potent activators of osteoclast-mediated bone destruction and significantly reduces the progression of osteolytic bone metastases. Moreover, functional blockade of LPA action on its cognate receptor LPA(1) using a pharmacological antagonist mimics the effects of silencing LPA(1) in tumor cells in vitro and substantially reduces bone metastasis progression in animals. Overall, these results suggest that inhibition of platelet-derived LPA action on LPA(1) expressed by tumor cells may be a promising therapeutic target for patients with bone metastases.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
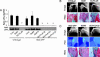



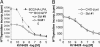

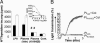
References
-
- Mundy G. R. Nat. Rev. Cancer. 2002;2:584–593. - PubMed
-
- Hillner B. E., Ingle J. N., Berenson J. R., Janjan N. A., Albain K. S., Lipton A., Yee G., Biermann J. S., Chlebowski R. T., Pfister D. G. J. Clin. Oncol. 2000;18:1378–1391. - PubMed
-
- Aoki J., Taira A., Takanezawa Y., Kishi Y., Hama K., Kishimoto T., Mizuno K., Saku K., Taguchi R., Arai H. J. Biol. Chem. 2002;277:48737–48744. - PubMed
-
- An S., Dickens M. A., Bleu T., Hallmark O. G., Goetzl E. J. Biochem. Biophys. Res. Commun. 1997;231:619–622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

