The mutant leucine-zipper domain impairs both dimerization and suppressive function of Foxp3 in T cells
- PMID: 16769892
- PMCID: PMC1480458
- DOI: 10.1073/pnas.0600225103
The mutant leucine-zipper domain impairs both dimerization and suppressive function of Foxp3 in T cells
Abstract
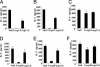
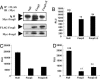
Regulatory T cells that express the Foxp3 transcription factor play important roles in preventing autoimmune diseases. Although several studies have demonstrated that the lack of the forkhead DNA-binding domain of Foxp3 caused severe autoimmune disease in scurfy mutant mice, the other functional domains of Foxp3 are less well characterized. Here, we show that the deletion of glutamic acid (DeltaE250) in the leucine-zipper domain of Foxp3 causes a loss of hyporesponsiveness when compared with wild-type Foxp3 upon antigenic stimulation. CD4 T cells that ectopically express the glutamic acid mutant show significant losses of suppressor activity both in vitro and in vivo. We also demonstrate that regulation of both Th1- and Th2-type cytokine secretion in CD4 T cells that express wild-type Foxp3 is significantly altered by the deletion of glutamic acid. Defects are also observed in the expression of adhesion molecules, such as l-selectin (CD62L) and CD103, suggesting an important role of glutamic acid in the migratory behavior of regulatory T cells. Finally, this mutation reduces transcriptional repressor activity and impairs the homodimerization of Foxp3. Taken together, our results provide insight into the mechanism that controls autoimmune diseases via the deletion of this single glutamic acid residue in the leucine-zipper domain of Foxp3.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

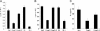
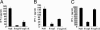

References
-
- Sakaguchi S. Annu. Rev. Immunol. 2004;22:531–562. - PubMed
-
- Gavin M., Rudensky A. Curr. Opin. Immunol. 2003;15:690–696. - PubMed
-
- Baecher-Allan C., Viglietta V., Hafler D. A. Semin. Immunol. 2004;16:89–98. - PubMed
-
- Schubert L. A., Jeffery E., Zhang Y., Ramsdell F., Ziegler S. F. J. Biol. Chem. 2001;276:37672–37679. - PubMed
-
- Hori S., Nomura T., Sakaguchi S. Science. 2003;299:1057–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

