A Mediator-responsive form of metazoan RNA polymerase II
- PMID: 16769904
- PMCID: PMC1480437
- DOI: 10.1073/pnas.0603702103
A Mediator-responsive form of metazoan RNA polymerase II
Abstract
RNA polymerase II (Pol II), whose 12 subunits are conserved across eukaryotes, is at the heart of the machinery responsible for transcription of mRNA. Although associated general transcription factors impart promoter specificity, responsiveness to gene- and tissue-selective activators additionally depends on the multiprotein Mediator coactivator complex. We have isolated from tissue extracts a distinct and abundant mammalian Pol II subpopulation that contains an additional tightly associated polypeptide, Gdown1. Our results establish that Gdown1-containing Pol II, designated Pol II(G), is selectively dependent on and responsive to Mediator. Thus, in an in vitro assay with general transcription factors, Pol II lacking Gdown1 displays unfettered levels of activator-dependent transcription in the presence or absence of Mediator. In contrast, Pol II(G) is dramatically less efficient in responding to activators in the absence of Mediator yet is highly and efficiently responsive to activators in the presence of Mediator. Our results reveal a transcriptional control mechanism in which Mediator-dependent regulation is enforced by means of Gdown1, which likely restricts Pol II function only to be reversed by Mediator.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
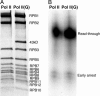

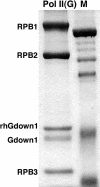
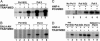
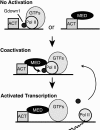
References
-
- Roeder R. G. FEBS Lett. 2005;579:909–915. - PubMed
-
- Roeder R. G. In: RNA Polymerase. Losick R., Chamberlain M., editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1976. pp. 285–329.
-
- Young R. A. Annu. Rev. Biochem. 1991;60:689–715. - PubMed
-
- Lee T. I., Young R. A. Annu. Rev. Genet. 2000;34:77–137. - PubMed
-
- Gnatt A. L., Cramer P., Fu J., Bushnell D. A., Kornberg R. D. Science. 2001;292:1876–1882. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

