The clone size of peripheral CD8 T cells is regulated by TCR promiscuity
- PMID: 16769997
- PMCID: PMC2118336
- DOI: 10.1084/jem.20052174
The clone size of peripheral CD8 T cells is regulated by TCR promiscuity
Abstract
Positive selection in the thymus and peripheral T cell survival depend on T cell receptor (TCR)-major histocompatibility complex (MHC) interactions, but it is not yet clear if both events follow exactly the same rules. We studied peripheral T cell survival and clone sizes in conditions of progressive reduction of restricting MHC-bearing cells or progressive ablation of different MHC molecules. Different CD8(+) T cell clones/polyclonal populations showed different survival and/or lymphopenia-driven proliferation requirements. We could correlate clone sizes to the capacity of each TCR to interact with different types of MHC complexes. Thus, although repertoire selection in the thymus is mainly conditioned by the affinity of TCR-MHC interactions, peripheral selection is determined by TCR cross-reactivity to environmental ligands.
Figures



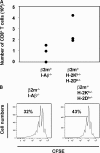
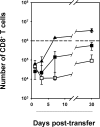
References
-
- Tanchot, C., F.A. Lemonnier, B. Perarnau, A.A. Freitas, and B. Rocha. 1997. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 276:2057–2062. - PubMed
-
- Murali-Krishna, K., L.L. Lau, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 286:1377–1381. - PubMed
-
- Witherden, D., N. van Oers, C. Waltzinger, A. Weiss, C. Benoist, and D. Mathis. 2000. Tetracycline-controllable selection of CD4(+) T cells: half-life and survival signals in the absence of major histocompatibility complex class II molecules. J. Exp. Med. 191:355–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

