Posttranslational modifications in Cu,Zn-superoxide dismutase and mutations associated with amyotrophic lateral sclerosis
- PMID: 16771675
- PMCID: PMC1633719
- DOI: 10.1089/ars.2006.8.847
Posttranslational modifications in Cu,Zn-superoxide dismutase and mutations associated with amyotrophic lateral sclerosis
Abstract
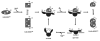
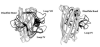
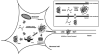
Activation of the enzyme Cu,Zn-superoxide dismutase (SOD1) involves several posttranslational modifications including copper and zinc binding, as well as formation of the intramolecular disulfide bond. The copper chaperone for SOD1, CCS, is responsible for intracellular copper loading in SOD1 under most physiological conditions. Recent in vitro and in vivo assays reveal that CCS not only delivers copper to SOD1 under stringent copper limitation, but it also facilitates the stepwise conversion of the disulfide-reduced immature SOD1 to the active disulfide-containing enzyme. The two new functions attributed to CCS, (i.e., O(2)-dependent sulfhydryl oxidase- and disulfide isomerase-like activities) indicate that this protein has attributes of the larger class of molecular chaperones. The CCS-dependent activation of SOD1 is dependent upon oxygen availability, suggesting that the cell only loads copper and activates this enzyme when O(2)-based oxidative stress is present. Thiol/disulfide status as well as metallation state of SOD1 significantly affects its structure and protein aggregation, which are relevant in pathologies of a neurodegenerative disease, amyotrophic lateral sclerosis (ALS). The authors review here a mechanism for posttranslational activation of SOD1 and discuss models for ALS in which the most immature forms of the SOD1 polypeptide exhibits propensity to form toxic aggregates.
Figures
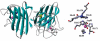



References
-
- Abernethy JL, Steinman HM, Hill RL. Bovine erythrocyte superoxide dismutase. Subunit structure and sequence location of the intrasubunit disulfide bond. J Biol Chem. 1974;249:7339–7347. - PubMed
-
- Andreassen OA, Dedeoglu A, Klivenyi P, Beal MF, Bush AI. N-acetyl-l-cysteine improves survival and preserves motor performance in an animal model of familial amyotrophic lateral sclerosis. Neuroreport. 2000;11:2491–2493. - PubMed
-
- Aoki M, Ogasawara M, Matsubara Y, Narisawa K, Nakamura S, Itoyama Y, Abe K. Mild ALS in Japan associated with novel SOD mutation. Nat Genet. 1993;5:323–324. - PubMed
-
- Arai K, Iizuka S, Tada Y, Oikawa K, Taniguchi N. Increase in the glucosylated form of erythrocyte Cu-Zn-superoxide dismutase in diabetes and close association of the nonenzymatic glucosylation with the enzyme activity. Biochim Biophys Acta. 1987;924:292–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

