Dependence on p38 MAPK signalling in the up-regulation of TLR2, TLR4 and TLR9 gene expression in Trichomonas vaginalis-treated HeLa cells
- PMID: 16771851
- PMCID: PMC1782292
- DOI: 10.1111/j.1365-2567.2006.02347.x
Dependence on p38 MAPK signalling in the up-regulation of TLR2, TLR4 and TLR9 gene expression in Trichomonas vaginalis-treated HeLa cells
Abstract
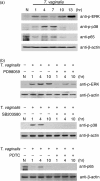
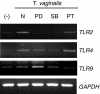
Toll-like receptors (TLRs) are pattern recognition receptors (PRRs) that recognize conserved pathogen-associated molecular patterns (PAMPs) synthesized by micro-organisms. Despite the essential requirement for TLRs in prokaryotic infection, the pattern and regulation of TLR gene expression by Trichomonas vaginalis in the mucocutaneous barrier are still unknown. Our hypothesis is that T. vaginalis-infected epithelial cells are major effector cells in the skin barrier. These cells function as a central regulator of TLR gene expression, thus accelerating the process of barrier dysfunction via increased release of chemokines and proinflammatory cytokines. To test this hypothesis, RT-PCR was performed on TLRs, interleukin (IL)-8 and tumour necrosis factor (TNF)-alpha. Stimulation of HeLa cells by T. vaginalis was observed to up-regulate TLR2, 4 and 9 mRNA expression as well as that of IL-8 and TNF-alpha. To further clarify the molecular mechanism of barrier devastation triggered by these up-regulatory stimuli, we examined the profiles of extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-kappaB activation in HeLa cells using specific inhibitors. Interestingly, pretreatment of HeLa cells with the p38 MAPK inhibitor SB203580 demonstrated inhibition of T. vaginalis-induced up-regulation of TLR2, 4, and 9 mRNA expression. By contrast, inhibition of ERK or NF-kappaB activation failed to block T. vaginalis-induced up-regulation of TLR9 mRNA expression or TLR2 and TLR4 mRNA expression, respectively. In addition, pretreatment with SB203580 reduced epithelium-derived IL-8 and TNF-alpha release evoked by T. vaginalis. Our results show that T. vaginalis infection of the mucocutaneous barrier could up-regulate TLR2, 4 and 9 gene expression via the p38 MAPK signalling pathway in epithelial cells; this process then leads to modulation of p38 MAPK-dependent IL-8 and TNF-alpha release from the epithelium.
Figures


Similar articles
-
Leptospiral membrane proteins stimulate pro-inflammatory chemokines secretion by renal tubule epithelial cells through toll-like receptor 2 and p38 mitogen activated protein kinase.Nephrol Dial Transplant. 2006 Apr;21(4):898-910. doi: 10.1093/ndt/gfi316. Epub 2005 Dec 8. Nephrol Dial Transplant. 2006. PMID: 16339163
-
Heat shock up-regulates expression of Toll-like receptor-2 and Toll-like receptor-4 in human monocytes via p38 kinase signal pathway.Immunology. 2005 Apr;114(4):522-30. doi: 10.1111/j.1365-2567.2004.02112.x. Immunology. 2005. PMID: 15804289 Free PMC article.
-
TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via posttranscriptional mechanism.Am J Physiol Gastrointest Liver Physiol. 2003 Aug;285(2):G282-90. doi: 10.1152/ajpgi.00503.2002. Epub 2003 Apr 17. Am J Physiol Gastrointest Liver Physiol. 2003. PMID: 12702497
-
Inflammatory responses during trichomoniasis: The role of Toll-like receptors and inflammasomes.Parasite Immunol. 2023 Aug;45(8):e13000. doi: 10.1111/pim.13000. Epub 2023 Jun 20. Parasite Immunol. 2023. PMID: 37338019 Review.
-
Effects of Toll-like receptor 1 and 2 agonist Pam3CSK4 on uveal melanocytes and relevant experimental mouse model.Exp Eye Res. 2024 Feb;239:109749. doi: 10.1016/j.exer.2023.109749. Epub 2023 Dec 17. Exp Eye Res. 2024. PMID: 38113956 Review.
Cited by
-
Strategies for Prevention and Treatment of Trichomonas vaginalis Infections.Clin Microbiol Rev. 2017 Jul;30(3):811-825. doi: 10.1128/CMR.00109-16. Clin Microbiol Rev. 2017. PMID: 28539504 Free PMC article. Review.
-
The molecular mechanism of acute liver injury and inflammatory response induced by Concanavalin A.Mol Biomed. 2021 Aug 10;2(1):24. doi: 10.1186/s43556-021-00049-w. Mol Biomed. 2021. PMID: 35006454 Free PMC article.
-
Toll-like receptor 9 activation by CpG oligodeoxynucleotide 7909 enhances the radiosensitivity of A549 lung cancer cells via the p53 signaling pathway.Oncol Lett. 2018 Apr;15(4):5271-5279. doi: 10.3892/ol.2018.7916. Epub 2018 Jan 31. Oncol Lett. 2018. PMID: 29541253 Free PMC article.
-
IL-10 release by bovine epithelial cells cultured with Trichomonas vaginalis and Tritrichomonas foetus.Mem Inst Oswaldo Cruz. 2013 Feb;108(1):110-2. doi: 10.1590/s0074-02762013000100018. Mem Inst Oswaldo Cruz. 2013. PMID: 23440124 Free PMC article.
-
Modulation of dendritic cell responses by parasites: a common strategy to survive.J Biomed Biotechnol. 2010;2010:357106. doi: 10.1155/2010/357106. Epub 2010 Feb 24. J Biomed Biotechnol. 2010. PMID: 20204070 Free PMC article. Review.
References
-
- Arroyo R, Engbring J, Alderete JF. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol. 1992;6:853–62. - PubMed
-
- Arici A, MacDonald PC, Casey ML. Regulation of monocyte chemotactic protein-1 gene expression in human endometrial cells in cultures. Mol Cell Endocrinol. 1995;107:189–97. - PubMed
-
- Arici A, Head JR, MacDonald PC, Casey ML. Regulation of interleukin-8 gene expression in human endometrial cells in culture. Mol Cell Endocrinol. 1993;94:195–204. - PubMed
-
- Laird SM, Li TC, Bolton AE. The production of placental protein 14 and interleukin 6 by human endometrial cells in culture. Hum Reprod. 1993;8:793–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

