Natural killer cells prime the responsiveness of autologous CD4+ T cells to CTLA4-Ig and interleukin-10 mediated inhibition in an allogeneic dendritic cell-mixed lymphocyte reaction
- PMID: 16771856
- PMCID: PMC1782277
- DOI: 10.1111/j.1365-2567.2006.02359.x
Natural killer cells prime the responsiveness of autologous CD4+ T cells to CTLA4-Ig and interleukin-10 mediated inhibition in an allogeneic dendritic cell-mixed lymphocyte reaction
Abstract
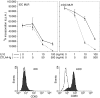
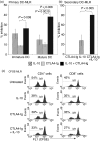
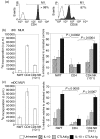
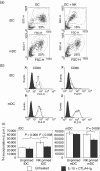
Cytotoxic T-lymphocyte antigen 4 immunoglobulin (CTLA4-Ig) and interleukin (IL)-10 are immunomodulatory molecules which target CD28 costimulation by acting either directly or indirectly on the CD80/86 receptors on dendritic cells (DCs). This study examined the effect of combined treatment with CTLA4-Ig and IL-10 on T-cell responsiveness in a dendritic cell-mixed lymphocyte reaction (DC-MLR). T cells derived from nylon wool enrichment (NWT cells) demonstrated 15% (P = 0.006) and 10% (P = 0.0015) inhibition of proliferation with suboptimal doses of IL-10 (5 ng/ml) and CTLA4-Ig (20 ng/ml), respectively. Combined treatment with both agents resulted in 38% inhibition (P = 0.004) of the MLR response compared with untreated controls. In contrast to NWT cells, which consisted of CD4+, CD8+ and CD56+ (NK) cells, purified CD4+ T cells were less responsive to immunomodulation by CTLA4-Ig and IL-10. Repletion of the CD4+ T cells with NK cells restored IL-10 and CTLA4-Ig mediated immunomodulation, suggesting a role for NK cells in the regulation of DC-T-cell interactions. The specific effect of NK cells on DC activation was demonstrated by CD80 up-regulation on DCs in the absence of T cells. However, in the absence of DCs, NK cells augmented the proliferation of autologous CD4+ T cells stimulated by anti-CD3 monoclonal antibody (mAb), which was blocked by CTLA4-Ig. It is proposed that, in the MLR, immunomodulation by suboptimal CTLA4-Ig and IL-10 is influenced by cellular interactions of NK cells with DCs and T cells involving DC lysis and costimulation. Thus, NK cells prime both DCs and T cells to low doses of CTLA4-Ig and IL-10 during alloimmune responses, providing evidence for the potential interaction between innate and adaptive immunity.
Figures
Similar articles
-
Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28- T cells and inhibit both T-cell proliferation and CTL function.Hum Immunol. 2004 Feb;65(2):142-56. doi: 10.1016/j.humimm.2003.12.001. Hum Immunol. 2004. PMID: 14969769
-
Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase.Int Immunol. 2004 Oct;16(10):1391-401. doi: 10.1093/intimm/dxh140. Epub 2004 Sep 6. Int Immunol. 2004. PMID: 15351783
-
Antigen-presenting T cells induce the development of cytotoxic CD4+ T cells. I. Involvement of the CD80-CD28 adhesion molecules.J Immunol. 1995 Jul 1;155(1):118-27. J Immunol. 1995. PMID: 7541409
-
NK cell regulation of T cell-mediated responses.Mol Immunol. 2005 Feb;42(4):451-4. doi: 10.1016/j.molimm.2004.07.025. Mol Immunol. 2005. PMID: 15607797 Review.
-
NK cells interactions with dendritic cells shape innate and adaptive immunity.Front Biosci. 2008 May 1;13:6443-54. doi: 10.2741/3165. Front Biosci. 2008. PMID: 18508671 Review.
Cited by
-
Differential Roles of Dendritic Cells in Expanding CD4 T Cells in Sepsis.Biomedicines. 2019 Jul 18;7(3):52. doi: 10.3390/biomedicines7030052. Biomedicines. 2019. PMID: 31323786 Free PMC article.
-
Early exposure of interferon-γ inhibits signal transducer and activator of transcription-6 signalling and nuclear factor κB activation in a short-term monocyte-derived dendritic cell culture promoting 'FAST' regulatory dendritic cells.Clin Exp Immunol. 2012 Mar;167(3):447-58. doi: 10.1111/j.1365-2249.2011.04537.x. Clin Exp Immunol. 2012. PMID: 22288588 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

