Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury
- PMID: 16772606
- PMCID: PMC1895564
- DOI: 10.1182/blood-2006-04-017251
Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury
Abstract
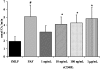
Transfusion-related acute lung injury (TRALI) is a form of posttransfusion acute pulmonary insufficiency that has been linked to the infusion of biologic response modifiers (BRMs), including antileukocyte antibodies and lipids. Soluble CD40 ligand (sCD40L) is a platelet-derived proinflammatory mediator that accumulates during platelet storage. We hypothesized that human polymorpho-nuclear leukocytes (PMNs) express CD40, CD40 ligation rapidly primes PMNs, and sCD40L induces PMN-mediated cytotoxicity of human pulmonary microvascular endothelial cells (HMVECs). Levels of sCD40L were measured in blood components and in platelet concentrates (PCs) implicated in TRALI or control PCs that did not elicit a transfusion reaction. All blood components contained higher levels of sCD40L than fresh plasma, with apheresis PCs evidencing the highest concentration of sCD40L followed by PCs from whole blood, whole blood, and packed red blood cells (PRBCs). PCs implicated in TRALI reactions contained significantly higher sCD40L levels than control PCs. PMNs express functional CD40 on the plasma membrane, and recombinant sCD40L (10 ng/mL-1 mug/mL) rapidly (5 minutes) primed the PMN oxidase. Soluble CD40L promoted PMN-mediated cytotoxicity of HMVECs as the second event in a 2-event in vitro model of TRALI. We concluded that sCD40L, which accumulates during blood component storage, has the capacity to activate adherent PMNs, causing endothelial damage and possibly TRALI in predisposed patients.
Figures
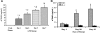


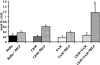

References
-
- Harnett MM. CD40: a growing cytoplasmic tale. Sci STKE. 2004;2004: e25. - PubMed
-
- Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92: 1041-1048. - PubMed
-
- Phipps RP, Kaufman J, Blumberg N. Platelet derived CD154 (CD40 ligand) and febrile responses to transfusion. Lancet. 2001;357: 2023-2024. - PubMed
-
- Phipps RP, Koumas L, Leung E, et al. The CD40-CD40 ligand system: a potential therapeutic target in atherosclerosis. Curr Opin Invest Drugs. 2001;2: 773-777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

