Beneficial effects of extended growth hormone treatment after hospital discharge in pediatric burn patients
- PMID: 16772783
- PMCID: PMC1479605
- DOI: 10.1097/01.sla.0000219676.69331.fd
Beneficial effects of extended growth hormone treatment after hospital discharge in pediatric burn patients
Abstract
Objective: To study the efficacy of growth hormone given to severely burned children from discharge to 12 months after burn and for 12 months after the drug was discontinued.
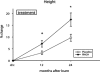
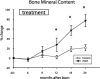
Summary background data: We have previously shown that low-dose recombinant human growth hormone (rhGH), given to children after a severe thermal injury, successfully improved lean muscle mass, bone mineral content, and growth. The aim of the present study was to investigate long-term functional improvements after treatment.
Methods: Forty-four pediatric patients with over 40% total body surface area burns were studied for 24 months after burn. Patients were randomized to receive either rhGH (0.05 mg/kg body weight) or placebo. Height, weight, body composition, serum hormones, resting energy expenditure, cardiac function, muscle strength, and number of reconstructive procedures performed were measured during rhGH treatment and for 12 months after treatment was discontinued. Statistical analysis used Tukey's multiple comparison test. Significance was accepted at P < 0.05.
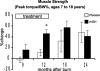
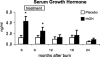
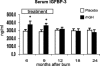
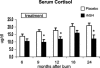
Results: Height, weight, lean body mass, bone mineral content, cardiac function, and muscle strength significantly improved during rhGH treatment compared with placebo (P < 0.05). This treatment significantly increased GH, IGF-I, and IGFBP-3, whereas serum cortisol decreased (P < 0.05). The number of operative reconstructive procedures was significantly lower with rhGH (P < 0.05). Improvements in height, bone mineral content, and IGF-1 concentrations persisted after rhGH treatment (P < 0.05). No side effects with rhGH were observed.
Conclusions: Administration of rhGH for 1 year after burn was safe and improved recovery. These salutary effects continued after rhGH treatment was discontinued.
Figures


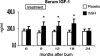
References
-
- Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1900. - PubMed
-
- Klein GL, Herndon DN, Langman CB, et al. Long-term reduction in bone mass following severe burn injury in children. J Pediatr. 1995;126:252–256. - PubMed
-
- Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg. 1990;125:392–395. - PubMed
-
- Wilmore D, Aulick L. Metabolic changes in burned patients. Surg Clin North Am. 1978;58:1173–1280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

